This is a preprint.
Transgenerational Epigenetic Inheritance of MHC Class I Gene Expression is Regulated by the CCAAT Promoter Element
- PMID: 37333336
- PMCID: PMC10274869
- DOI: 10.1101/2023.04.13.536772
Transgenerational Epigenetic Inheritance of MHC Class I Gene Expression is Regulated by the CCAAT Promoter Element
Update in
-
CCAAT Promoter element regulates transgenerational expression of the MHC class I gene.Chromosoma. 2024 Jul;133(3):203-216. doi: 10.1007/s00412-024-00820-2. Epub 2024 Jun 26. Chromosoma. 2024. PMID: 38922437 Free PMC article.
Abstract
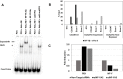
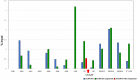
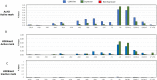
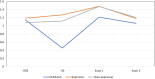
Transgenerational epigenetic inheritance is defined as the transmission of traits or gene expression patterns across multiple generations that do not derive from DNA alterations. The effect of multiple stress factors or metabolic changes resulting in such inheritance have been documented in plants, worms and flies and mammals. The molecular basis for epigenetic inheritance has been linked to histone and DNA modifications and non-coding RNA. In this study, we show that mutation of a promoter element, the CCAAT box, disrupts stable expression of an MHC Class I transgene, resulting in variegated expression among progeny for at least 4 generations in multiple independently derived transgenic lines. Histone modifications and RNA polII binding correlate with expression, whereas DNA methylation and nucleosome occupancy do not. Mutation of the CCAAT box abrogates NF-Y binding and results in changes to CTCF binding and DNA looping patterns across the gene that correlate with expression status from one generation to the next. These studies identify the CCAAT promoter element as a regulator of stable transgenerational epigenetic inheritance. Considering that the CCAAT box is present in 30% of eukaryotic promoters, this study could provide important insights into how fidelity of gene expression patterns is maintained through multiple generations.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURE The authors have no conflicts of interest to disclose.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
