Organic and inorganic nutrients modulate taxonomic diversity and trophic strategies of small eukaryotes in oligotrophic oceans
- PMID: 37333435
- PMCID: PMC10117809
- DOI: 10.1093/femsmc/xtac029
Organic and inorganic nutrients modulate taxonomic diversity and trophic strategies of small eukaryotes in oligotrophic oceans
Abstract
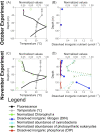
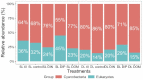
As the oligotrophic gyres expand due to global warming, exacerbating resource limitation impacts on primary producers, predicting changes to microbial assemblages and productivity requires knowledge of the community response to nutrient availability. This study examines how organic and inorganic nutrients influence the taxonomic and trophic composition (18S metabarcoding) of small eukaryotic plankton communities (< 200 µm) within the euphotic zone of the oligotrophic Sargasso Sea. The study was conducted by means of field sampling of natural microbial communities and laboratory incubation of these communities under different nutrient regimes. Dissimilarity in community composition increased along a depth gradient, with a homogeneous protist community within the mixed layer and distinct microbial assemblages at different depths below the deep chlorophyll maximum. A nutrient enrichment assay revealed the potential of natural microbial communities to rapidly shift in composition in response to nutrient addition. Results highlighted the importance of inorganic phosphorus availability, largely understudied compared to nitrogen, in constraining microbial diversity. Dissolved organic matter addition led to a loss of diversity, benefiting a limited number of phagotrophic and mixotrophic taxa. Nutrient history of the community sets the physiological responsiveness of the eukaryotic community to changing nutrient regimes and needs to be considered in future studies.
Keywords: biodiversity; dissolved inorganic phosphorus; marine protists; metabarcoding; nutrient competition; trophic strategy.
© The Author(s) 2022. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
None declared
Figures









References
-
- Agis M, Granda A, Dolan JR. A cautionary note: examples of possible microbial community dynamics in dilution grazing experiments. J Exp Mar Biol Ecol. 2007;341:176–83.
-
- Antia NJ, Harrison PJ, Oliveira L. The role of dissolved organic nitrogen in phytoplankton nutrition, cell biology and ecology. Phycologia. 1991;30:1–89.
-
- Arrigo KR. Marine microorganisms and global nutrient cycles. Nature. 2005;437:349–55. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
