A distinct Golgi-targeting mechanism of dGM130 in Drosophila neurons
- PMID: 37333614
- PMCID: PMC10272413
- DOI: 10.3389/fnmol.2023.1206219
A distinct Golgi-targeting mechanism of dGM130 in Drosophila neurons
Abstract
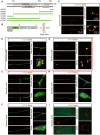
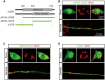
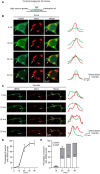
GM130 is a matrix protein that is conserved in metazoans and involved in the architecture of the Golgi apparatus. In neurons, Golgi apparatus and dendritic Golgi outposts (GOs) have different compartmental organizations, and GM130 localization is present in both, indicating that GM130 has a unique Golgi-targeting mechanism. Here, we investigated the Golgi-targeting mechanism of the GM130 homologue, dGM130, using in vivo imaging of Drosophila dendritic arborization (da) neurons. The results showed that two independent Golgi-targeting domains (GTDs) with different Golgi localization characteristics in dGM130, together determined the precise localization of dGM130 in both the soma and dendrites. GTD1, covering the first coiled-coil region, preferentially targeted to somal Golgi rather than GOs; whereas GTD2, containing the second coiled-coil region and C-terminus, dynamically targeted to Golgi in both soma and dendrites. These findings suggest that there are two distinct mechanisms by which dGM130 targets to the Golgi apparatus and GOs, underlying the structural differences between them, and further provides new insights into the formation of neuronal polarity.
Keywords: GM130; Golgi apparatus; Golgi-targeting domains; dendrites; neuronal polarity.
Copyright © 2023 Cheng, Chang, Gong and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
GM130 is required for compartmental organization of dendritic golgi outposts.Curr Biol. 2014 Jun 2;24(11):1227-33. doi: 10.1016/j.cub.2014.04.008. Epub 2014 May 15. Curr Biol. 2014. PMID: 24835455 Free PMC article.
-
Golgi Outposts Locally Regulate Microtubule Orientation in Neurons but Are Not Required for the Overall Polarity of the Dendritic Cytoskeleton.Genetics. 2020 Jun;215(2):435-447. doi: 10.1534/genetics.119.302979. Epub 2020 Apr 7. Genetics. 2020. PMID: 32265236 Free PMC article.
-
A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins.Curr Biol. 1999 Apr 8;9(7):381-4. doi: 10.1016/s0960-9822(99)80167-5. Curr Biol. 1999. PMID: 10209123
-
Emerging new roles of GM130, a cis-Golgi matrix protein, in higher order cell functions.J Pharmacol Sci. 2010;112(3):255-64. doi: 10.1254/jphs.09r03cr. Epub 2010 Mar 2. J Pharmacol Sci. 2010. PMID: 20197635 Review.
-
Local Secretory Trafficking Pathways in Neurons and the Role of Dendritic Golgi Outposts in Different Cell Models.Front Mol Neurosci. 2020 Nov 26;13:597391. doi: 10.3389/fnmol.2020.597391. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33324160 Free PMC article. Review.
Cited by
-
The inter-organelle cross-talk finely orchestrated in the amyloidogenic processing of amyloid precursor protein in dendritic arborization neurons of Drosophila.Theranostics. 2025 Feb 10;15(7):2951-2966. doi: 10.7150/thno.104345. eCollection 2025. Theranostics. 2025. PMID: 40083942 Free PMC article.
References
-
- Bhat G., Hothpet V. R., Lin M. F., Cheng P. W. (2017). Shifted Golgi targeting of glycosyltransferases and alpha-mannosidase IA from giantin to GM130-GRASP65 results in formation of high mannose N-glycans in aggressive prostate cancer cells. Biochim. Biophys. Acta Gen. Subj. 1861, 2891–2901. doi: 10.1016/j.bbagen.2017.08.006 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

