Unlocking antagonistic potential of Bacillus amyloliquefaciens KRS005 to control gray mold
- PMID: 37333651
- PMCID: PMC10272387
- DOI: 10.3389/fmicb.2023.1189354
Unlocking antagonistic potential of Bacillus amyloliquefaciens KRS005 to control gray mold
Abstract
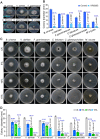
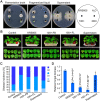
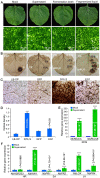
To establish a safe, efficient, and simple biocontrol measure for gray mold disease caused by Botrytis cinerea, the basic characteristics and antifungal activity of KRS005 were studied from multiple aspects including morphological observation, multilocus sequence analysis and typing (MLSA-MLST), physical-biochemical assays, broad-spectrum inhibitory activities, control efficiency of gray mold, and determination of plant immunity. The strain KRS005, identified as Bacillus amyloliquefaciens, demonstrated broad-spectrum inhibitory activities against various pathogenic fungi by dual confrontation culture assays, of which the inhibition rate of B. cinerea was up to 90.3%. Notably, through the evaluation of control efficiency, it was found that KRS005 fermentation broth could effectively control the occurrence of tobacco leaves gray mold by determining the lesion diameter and biomass of B. cinerea on tobacco leaves still had a high control effect after dilution of 100 folds. Meanwhile, KRS005 fermentation broth had no impact on the mesophyll tissue of tobacco leaves. Further studies showed that plant defense-related genes involved in reactive oxygen species (ROS), salicylic acid (SA), and jasmonic acid (JA)-related signal pathways were significantly upregulated when tobacco leaves were sprayed with KRS005 cell-free supernatant. In addition, KRS005 could inhibit cell membrane damage and increase the permeability of B. cinerea. Overall, KRS005, as a promising biocontrol agent, would likely serve as an alternative to chemical fungicides to control gray mold.
Keywords: Bacillus amyloliquefaciens; Botrytis cinerea; biological control; gray mold; plant immunity.
Copyright © 2023 Qi, Wang, Han, Song, Ali, Dai, Zhang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Insights into the Biocontrol Function of a Burkholderia gladioli Strain against Botrytis cinerea.Microbiol Spectr. 2023 Mar 2;11(2):e0480522. doi: 10.1128/spectrum.04805-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36861984 Free PMC article.
-
The Involvement of Jasmonic Acid, Ethylene, and Salicylic Acid in the Signaling Pathway of Clonostachys rosea-Induced Resistance to Gray Mold Disease in Tomato.Phytopathology. 2019 Jul;109(7):1102-1114. doi: 10.1094/PHYTO-01-19-0025-R. Epub 2019 Jun 10. Phytopathology. 2019. PMID: 30880572
-
Biocontrol potential of Pseudomonas protegens ML15 against Botrytis cinerea causing gray mold on postharvest tomato (Solanum lycopersicum var. cerasiforme).Front Plant Sci. 2023 Dec 7;14:1288408. doi: 10.3389/fpls.2023.1288408. eCollection 2023. Front Plant Sci. 2023. PMID: 38143572 Free PMC article.
-
Characterization of a new Bacillus velezensis as a powerful biocontrol agent against tomato gray mold.Pestic Biochem Physiol. 2022 Oct;187:105199. doi: 10.1016/j.pestbp.2022.105199. Epub 2022 Aug 8. Pestic Biochem Physiol. 2022. PMID: 36127070
-
Characterization of Volatile Organic Compounds Produced by Bacillus siamensis YJ15 and Their Antifungal Activity Against Botrytis cinerea.Plant Dis. 2022 Sep;106(9):2321-2329. doi: 10.1094/PDIS-01-22-0230-RE. Epub 2022 Aug 10. Plant Dis. 2022. PMID: 35380464
Cited by
-
Plant Growth Promotion and Plant Disease Suppression Induced by Bacillus amyloliquefaciens Strain GD4a.Plants (Basel). 2024 Feb 28;13(5):672. doi: 10.3390/plants13050672. Plants (Basel). 2024. PMID: 38475518 Free PMC article.
-
Bacillus lumedeiriae sp. nov., a Gram-Positive, Spore-Forming Rod Isolated from a Pharmaceutical Facility Production Environment and Added to the MALDI Biotyper® Database.Microorganisms. 2024 Dec 5;12(12):2507. doi: 10.3390/microorganisms12122507. Microorganisms. 2024. PMID: 39770710 Free PMC article.
-
Insights into the biocontrol and plant growth promotion functions of Bacillus altitudinis strain KRS010 against Verticillium dahliae.BMC Biol. 2024 May 20;22(1):116. doi: 10.1186/s12915-024-01913-1. BMC Biol. 2024. PMID: 38764012 Free PMC article.
References
-
- Basit A., Farhan M., Abbas M., Wang Y., Zhao D. G., Mridha A. U., et al. . (2021). Do microbial protein elicitors PeaT1 obtained from Alternaria tenuissimaand PeBL1 from Brevibacillus laterosporusenhance defense response against tomato aphid (Myzus persicae). Saudi J Biol Sci. 28, 3242–3248. doi: 10.1016/j.sjbs.2021.02.063, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

