Promoted Abutment-Soft Tissue Integration Around Self-Glazed Zirconia Surfaces with Nanotopography Fabricated by Additive 3D Gel Deposition
- PMID: 37333732
- PMCID: PMC10276606
- DOI: 10.2147/IJN.S404047
Promoted Abutment-Soft Tissue Integration Around Self-Glazed Zirconia Surfaces with Nanotopography Fabricated by Additive 3D Gel Deposition
Abstract
Introduction: Improving the biological sealing around dental abutments could promote the long-term success of implants. Although titanium abutments have a wide range of clinical applications, they incur esthetic risks due to their color, especially in the esthetic zone. Currently, zirconia has been applied as an esthetic alternative material for implant abutments; however, zirconia is purported to be an inert biomaterial. How to improve the biological activities of zirconia has thus become a popular research topic. In this study, we presented a novel self-glazed zirconia (SZ) surface with nanotopography fabricated by additive 3D gel deposition and investigated its soft tissue integration capability compared to that of clinically used titanium and polished conventional zirconia surfaces.
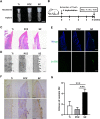
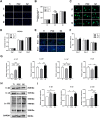
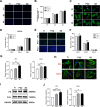
Materials and methods: Three groups of disc samples were prepared for in vitro study and the three groups of abutment samples were prepared for in vivo study. The surface topography, roughness, wettability and chemical composition of the samples were examined. Moreover, we analyzed the effect of the three groups of samples on protein adsorption and on the biological behavior of human gingival keratinocytes (HGKs) and human gingival fibroblasts (HGFs). Furthermore, we conducted an in vivo study in which the bilateral mandibular anterior teeth of rabbits were extracted and replaced with implants and corresponding abutments.
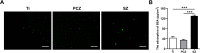
Results: The surface of SZ showed a unique nanotopography with nm range roughness and a greater ability to absorb protein. The promoted expression of adhesion molecules in both HGKs and HGFs was observed on the SZ surface compared to the surfaces of Ti and PCZ, while the cell viability and proliferation of HGKs and the number of HGFs adhesion were not significant among all groups. In vivo results showed that the SZ abutment formed strong biological sealing at the abutment-soft tissue interface and exhibited markedly more hemidesmosomes when observed with a transmission electron microscope.
Conclusion: These results demonstrated that the novel SZ surface with nanotopography promoted soft tissue integration, suggesting its promising application as a zirconia surface for the dental abutment.
Keywords: 3D gel deposition; adhesion molecule; dental abutment; nanotopography; self-glazed zirconia; soft tissue integration.
© 2023 Huang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

