NF-κB fingerprinting reveals heterogeneous NF-κB composition in diffuse large B-cell lymphoma
- PMID: 37333821
- PMCID: PMC10272839
- DOI: 10.3389/fonc.2023.1181660
NF-κB fingerprinting reveals heterogeneous NF-κB composition in diffuse large B-cell lymphoma
Abstract
Introduction: Improving treatments for Diffuse Large B-Cell Lymphoma (DLBCL) is challenged by the vast heterogeneity of the disease. Nuclear factor-κB (NF-κB) is frequently aberrantly activated in DLBCL. Transcriptionally active NF-κB is a dimer containing either RelA, RelB or cRel, but the variability in the composition of NF-κB between and within DLBCL cell populations is not known.
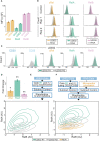
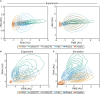
Results: Here we describe a new flow cytometry-based analysis technique termed "NF-κB fingerprinting" and demonstrate its applicability to DLBCL cell lines, DLBCL core-needle biopsy samples, and healthy donor blood samples. We find each of these cell populations has a unique NF-κB fingerprint and that widely used cell-of-origin classifications are inadequate to capture NF-κB heterogeneity in DLBCL. Computational modeling predicts that RelA is a key determinant of response to microenvironmental stimuli, and we experimentally identify substantial variability in RelA between and within ABC-DLBCL cell lines. We find that when we incorporate NF-κB fingerprints and mutational information into computational models we can predict how heterogeneous DLBCL cell populations respond to microenvironmental stimuli, and we validate these predictions experimentally.
Discussion: Our results show that the composition of NF-κB is highly heterogeneous in DLBCL and predictive of how DLBCL cells will respond to microenvironmental stimuli. We find that commonly occurring mutations in the NF-κB signaling pathway reduce DLBCL's response to microenvironmental stimuli. NF-κB fingerprinting is a widely applicable analysis technique to quantify NF-κB heterogeneity in B cell malignancies that reveals functionally significant differences in NF-κB composition within and between cell populations.
Keywords: DLCBL; NFkB; TME (tumor microenvironment); computational biology; lymphoma; math modeling; systems biology.
Copyright © 2023 Jayawant, Pack, Clark, Kennedy, Ghodke, Jones, Pepper, Pepper and Mitchell.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
ASB2 is a direct target of FLI1 that sustains NF-κB pathway activation in germinal center-derived diffuse large B-cell lymphoma.J Exp Clin Cancer Res. 2021 Nov 11;40(1):357. doi: 10.1186/s13046-021-02159-3. J Exp Clin Cancer Res. 2021. PMID: 34763718 Free PMC article.
-
Systems biology-enabled targeting of NF-κΒ and BCL2 overcomes microenvironment-mediated BH3-mimetic resistance in DLBCL.bioRxiv [Preprint]. 2025 Feb 1:2024.11.30.626166. doi: 10.1101/2024.11.30.626166. bioRxiv. 2025. Update in: Cell Death Dis. 2025 Aug 16;16(1):620. doi: 10.1038/s41419-025-07942-0. PMID: 39677808 Free PMC article. Updated. Preprint.
-
The alternative RelB NF-κB subunit is a novel critical player in diffuse large B-cell lymphoma.Blood. 2022 Jan 20;139(3):384-398. doi: 10.1182/blood.2020010039. Blood. 2022. PMID: 34232979
-
Molecular complexity of diffuse large B-cell lymphoma: a molecular perspective and therapeutic implications.J Appl Genet. 2024 Feb;65(1):57-72. doi: 10.1007/s13353-023-00804-5. Epub 2023 Nov 25. J Appl Genet. 2024. PMID: 38001281 Review.
-
Targeting MALT1 for the treatment of diffuse large B-cell lymphoma.Leuk Lymphoma. 2022 Apr;63(4):789-798. doi: 10.1080/10428194.2021.1999444. Epub 2021 Nov 16. Leuk Lymphoma. 2022. PMID: 34783281 Review.
Cited by
-
Research Progress Regarding the Effect and Mechanism of Dietary Polyphenols in Liver Fibrosis.Molecules. 2023 Dec 25;29(1):127. doi: 10.3390/molecules29010127. Molecules. 2023. PMID: 38202710 Free PMC article. Review.
-
NF-κB signaling driven by oncogenic Ras contributes to tumorigenesis in a Drosophila carcinoma model.PLoS Biol. 2025 Apr 28;23(4):e3002663. doi: 10.1371/journal.pbio.3002663. eCollection 2025 Apr. PLoS Biol. 2025. PMID: 40294135 Free PMC article.
-
Computational modelling of aggressive B-cell lymphoma.Biochem Soc Trans. 2025 Aug 29;53(4):723-735. doi: 10.1042/BST20253039. Biochem Soc Trans. 2025. PMID: 40613779 Free PMC article. Review.
-
Systems biology-enabled targeting of NF-κΒ and BCL2 overcomes microenvironment-mediated BH3-mimetic resistance in DLBCL.Cell Death Dis. 2025 Aug 16;16(1):620. doi: 10.1038/s41419-025-07942-0. Cell Death Dis. 2025. PMID: 40819126 Free PMC article.
References
LinkOut - more resources
Full Text Sources

