Lactonase-mediated inhibition of quorum sensing largely alters phenotypes, proteome, and antimicrobial activities in Burkholderia thailandensis E264
- PMID: 37333853
- PMCID: PMC10272358
- DOI: 10.3389/fcimb.2023.1190859
Lactonase-mediated inhibition of quorum sensing largely alters phenotypes, proteome, and antimicrobial activities in Burkholderia thailandensis E264
Abstract
Introduction: Burkholderia thailandensis is a study model for Burkholderia pseudomallei, a highly virulent pathogen, known to be the causative agent of melioidosis and a potential bioterrorism agent. These two bacteria use an (acyl-homoserine lactone) AHL-mediated quorum sensing (QS) system to regulate different behaviors including biofilm formation, secondary metabolite productions, and motility.
Methods: Using an enzyme-based quorum quenching (QQ) strategy, with the lactonase SsoPox having the best activity on B. thailandensis AHLs, we evaluated the importance of QS in B. thailandensis by combining proteomic and phenotypic analyses.
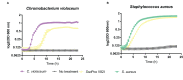
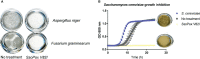
Results: We demonstrated that QS disruption largely affects overall bacterial behavior including motility, proteolytic activity, and antimicrobial molecule production. We further showed that QQ treatment drastically decreases B. thailandensis bactericidal activity against two bacteria (Chromobacterium violaceum and Staphylococcus aureus), while a spectacular increase in antifungal activity was observed against fungi and yeast (Aspergillus niger, Fusarium graminearum and Saccharomyces cerevisiae).
Discussion: This study provides evidence that QS is of prime interest when it comes to understanding the virulence of Burkholderia species and developing alternative treatments.
Keywords: Acyl-homoserine lactone; Burkholderia thailandensis; lactonase; quorum quenching; quorum sensing.
Copyright © 2023 Gonzales, Plener, Armengaud, Armstrong, Chabrière and Daudé.
Conflict of interest statement
MG, LP, ÉC, and DD report receiving personal fees from Gene&GreenTK during the study. ÉC, LP, and DD have filed the patents FR3093894, EP3941206, WO2020187861. ÉC, LP, and DD are shareholders in Gene&GreenTK. DD is CEO of Gene&GreenTK. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
ScmR, a Global Regulator of Gene Expression, Quorum Sensing, pH Homeostasis, and Virulence in Burkholderia thailandensis.J Bacteriol. 2020 Jun 9;202(13):e00776-19. doi: 10.1128/JB.00776-19. Print 2020 Jun 9. J Bacteriol. 2020. PMID: 32312745 Free PMC article.
-
The Complex Quorum Sensing Circuitry of Burkholderia thailandensis Is Both Hierarchically and Homeostatically Organized.mBio. 2017 Dec 5;8(6):e01861-17. doi: 10.1128/mBio.01861-17. mBio. 2017. PMID: 29208745 Free PMC article.
-
Two rsaM Homologues Encode Central Regulatory Elements Modulating Quorum Sensing in Burkholderia thailandensis.J Bacteriol. 2018 Jun 25;200(14):e00727-17. doi: 10.1128/JB.00727-17. Print 2018 Jul 15. J Bacteriol. 2018. PMID: 29507087 Free PMC article.
-
Acyl-homoserine lactone-based quorum sensing and quorum quenching hold promise to determine the performance of biological wastewater treatments: An overview.Chemosphere. 2016 Aug;157:137-51. doi: 10.1016/j.chemosphere.2016.05.032. Epub 2016 May 20. Chemosphere. 2016. PMID: 27213243 Review.
-
Quorum quenching: role in nature and applied developments.FEMS Microbiol Rev. 2016 Jan;40(1):86-116. doi: 10.1093/femsre/fuv038. Epub 2015 Oct 1. FEMS Microbiol Rev. 2016. PMID: 26432822 Review.
References
-
- Adler D., Kelly T. S., Elliot T., Adamson J. (2022) Vioplot: violin plot. Available at: https://github.com/TomKellyGenetics/vioplot.
-
- Arendrup M. C., Guinea J., Cuenca-Estrella M., Meletiadis J., Mouton J. W., Lagrou K., et al. . (2015). EUCAST definitive document E.Def.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

