Integration failure of regenerated limb tissue is associated with incongruencies in positional information in the Mexican axolotl
- PMID: 37333984
- PMCID: PMC10272535
- DOI: 10.3389/fcell.2023.1152510
Integration failure of regenerated limb tissue is associated with incongruencies in positional information in the Mexican axolotl
Abstract
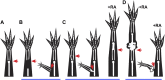
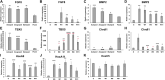
Introduction: Little is known about how the newly regenerated limb tissues in the Mexican axolotl seamlessly integrate with the remaining stump tissues to form a functional structure, and why this doesn't occur in some regenerative scenarios. In this study, we evaluate the phenomenological and transcriptional characteristics associated with integration failure in ectopic limb structures generated by treating anterior-located ectopic blastemas with Retinoic Acid (RA) and focusing on the "bulbus mass" tissue that forms between the ectopic limb and the host site. We additionally test the hypothesis that the posterior portion of the limb base contains anterior positional identities. Methods: The positional identity of the bulbus mass was evaluated by assaying regenerative competency, the ability to induce new pattern in the Accessory Limb Model (ALM) assay, and by using qRTPCR to quantify the relative expression of patterning genes as the bulbus mass deintegrates from the host site. We additionally use the ALM and qRTPCR to analyze the distribution of anterior and posterior positional identities along the proximal/distal limb axis of uninjured and regenerating limbs. Results: The bulbus mass regenerates limb structures with decreased complexity when amputated and is able to induce complex ectopic limb structure only when grafted into posterior-located ALMs. Expressional analysis shows significant differences in FGF8, BMP2, TBX5, Chrdl1, HoxA9, and HoxA11 expression between the bulbus mass and the host site when deintegration is occuring. Grafts of posterior skin from the distal limb regions into posterior ALMs at the base of the limb induce ectopic limb structures. Proximally-located blastemas express significantly less HoxA13 and Ptch1, and significantly more Alx4 and Grem1 than distally located blastemas. Discussion: These findings show that the bulbus mass has an anterior-limb identity and that the expression of limb patterning genes is mismatched between the bulbus mass and the host limb. Our findings additionally show that anterior positional information is more abundant at the limb base, and that anterior patterning genes are more abundantly expressed in proximally located blastemas compared to blastemas in the more distal regions of the limb. These experiments provide valuable insight into the underlying causes of integration failure and further map the distribution of positional identities in the mature limb.
Keywords: accessory limb model; axolotl; bulbous mass; integration; limb patterning; limb regeneration; retinoic acid.
Copyright © 2023 Vieira, Raymond, Kelley, Cherubino, Sahin and McCusker.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bodemer C. W. (1958). The development of nerve-induced supernumerary limbs in the adult newt, Triturus viridescens. J. Morphol. 102, 555–581. 10.1002/jmor.1051020304 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

