Steroid Sparing Maintenance Immunosuppression in Highly Sensitised Patients Receiving Alemtuzumab Induction
- PMID: 37334011
- PMCID: PMC10272412
- DOI: 10.3389/ti.2023.11056
Steroid Sparing Maintenance Immunosuppression in Highly Sensitised Patients Receiving Alemtuzumab Induction
Abstract
This analysis reports on the outcomes of two different steroid sparing immunosuppression protocols used in the management of 120 highly sensitised patients (HSPs) with cRF>85% receiving Alemtuzumab induction, 53 maintained on tacrolimus (FK) monotherapy and 67 tacrolimus plus mycophenolate mofetil (FK + MMF). There was no difference in the median cRF or mode of sensitisation between the two groups, although the FK + MMF cohort received more poorly matched grafts. There was no difference in one-year patient or allograft survival, however rejection free survival was inferior with FK monotherapy compared with FK + MMF at 65.4% and 91.4% respectively, p < 0.01. DSA-free survival was comparable. Whilst there was no difference in rates of BK between the cohorts, CMV-free survival was inferior in the FK + MMF group at 86.0% compared with 98.1% in the FK group, p = 0.026. One-year post-transplant diabetes free survival was 89.6% and 100.0% in the FK and FK + MMF group respectively, p = 0.027, the difference attributed to the use of prednisolone to treat rejection in the FK cohort, p = 0.006. We report good outcomes in HSPs utilising a steroid sparing protocol with Alemtuzumab induction and FK + MMF maintenance and provide granular data on immunological and infectious complications to inform steroid avoidance in these patient groups.
Keywords: Alemtuzumab; HLA; calculated reaction frequency; highly sensitised; steroid sparing.
Copyright © 2023 Santos, Spensley, Gunby, Clarke, Anand, Roufosse and Willicombe.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

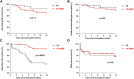
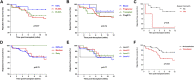

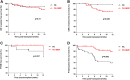
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

