Bimodal modulation of L1 interneuron activity in anterior cingulate cortex during fear conditioning
- PMID: 37334059
- PMCID: PMC10272719
- DOI: 10.3389/fncir.2023.1138358
Bimodal modulation of L1 interneuron activity in anterior cingulate cortex during fear conditioning
Abstract
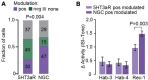
The anterior cingulate cortex (ACC) plays a crucial role in encoding, consolidating and retrieving memories related to emotionally salient experiences, such as aversive and rewarding events. Various studies have highlighted its importance for fear memory processing, but its circuit mechanisms are still poorly understood. Cortical layer 1 (L1) of the ACC might be a particularly important site of signal integration, since it is a major entry point for long-range inputs, which is tightly controlled by local inhibition. Many L1 interneurons express the ionotropic serotonin receptor 3a (5HT3aR), which has been implicated in post-traumatic stress disorder and in models of anxiety. Hence, unraveling the response dynamics of L1 interneurons and subtypes thereof during fear memory processing may provide important insights into the microcircuit organization regulating this process. Here, using 2-photon laser scanning microscopy of genetically encoded calcium indicators through microprisms in awake mice, we longitudinally monitored over days the activity of L1 interneurons in the ACC in a tone-cued fear conditioning paradigm. We observed that tones elicited responses in a substantial fraction of the imaged neurons, which were significantly modulated in a bidirectional manner after the tone was associated to an aversive stimulus. A subpopulation of these neurons, the neurogliaform cells (NGCs), displayed a net increase in tone-evoked responses following fear conditioning. Together, these results suggest that different subpopulations of L1 interneurons may exert distinct functions in the ACC circuitry regulating fear learning and memory.
Keywords: 2-photon laser scanning microscopy; anterior cingulate cortex; fear learning; microprism; neurogliaform cells; serotonin receptor 3a.
Copyright © 2023 Fossati, Kiss-Bodolay, Prados, Chéreau, Husi, Cadilhac, Gomez, Silva, Dayer and Holtmaat.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

