Comparison of Anti-rabies Virus Nucleoprotein IgY Prepared by DNA Immunization and Protein Immunization
- PMID: 37334105
- PMCID: PMC10270695
- DOI: 10.2141/jpsa.2023014
Comparison of Anti-rabies Virus Nucleoprotein IgY Prepared by DNA Immunization and Protein Immunization
Abstract
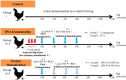
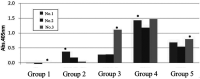
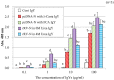
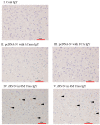
Immunization of egg-laying hens with viral antigens efficiently produces large amounts of virus-specific IgY antibodies from egg yolks. A supply of practical and economical antibodies against the rabies virus is being desired worldwide. We immunized hens with the antigen gene DNA of the rabies virus, purified specific IgY antibodies from the egg yolk, and characterized the immuno-protein chemistry for use as a diagnosis. To prepare specific IgY antibodies against rabies virus nucleoprotein (RV-N) by DNA immunization, laying hens were pre-injected with λ-carrageenan or Freund's complete adjuvant to increase local immune activity (pre-immune stimulation), and then immunized with RV-N recombinant plasmid DNA. RV-N-specific IgY antibodies were prepared from egg yolks of immunized hens. For comparison, conventional protein antigen immunization was also used to induce the production of RV-N-specific IgY antibodies. Laying hens were immunized with an RV-N protein antigen and RV-N-specific IgY was purified from egg yolks. The binding activity against RV-N antigens was examined using IgY samples prepared by DNA (with pre-immune stimulation) and protein immunization. Immunohistochemical staining showed that IgY antibodies prepared by protein immunization strongly detected viral antigens in the brain sections of dogs infected with the virus, whereas IgY antibodies prepared by DNA immunization did not. Enzyme-linked immunosorbent assay was performed using a commercially available rabies vaccine (inactivated virus) treated with 10% formalin and heating (60°C, 30 min and 90°C, 5 min). IgY prepared by DNA immunization had weaker reactivity with denatured antigens and lower antigen concentrations than IgY prepared by protein immunization. These results suggest that it is necessary to develop a DNA immunization method for inducing IgY antibodies against the rabies virus that strongly bind to native and denatured antigens to prepare specific IgYs that can be used for antigen detection in clinical tests.
Keywords: DNA immunization; FCA; IHC; IgY; Rabies virus; λ-carrageenan.
2023 Japan Poultry Science Association.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflict of interest.
Figures

Similar articles
-
Preparation of Anti-Rabies Virus N Protein IgYs by DNA Immunization of Hens Using Different Types of Adjuvants.J Poult Sci. 2022 Apr 25;59(2):191-196. doi: 10.2141/jpsa.0210053. J Poult Sci. 2022. PMID: 35528385 Free PMC article.
-
Egg yolk and serum antibody titers of broiler breeder hens immunized with uricase and or urease.Poult Sci. 2011 Oct;90(10):2162-8. doi: 10.3382/ps.2010-00855. Poult Sci. 2011. PMID: 21933996
-
Efficient Production of Human Norovirus-Specific IgY in Egg Yolks by Vaccination of Hens with a Recombinant Vesicular Stomatitis Virus Expressing VP1 Protein.Viruses. 2019 May 16;11(5):444. doi: 10.3390/v11050444. Viruses. 2019. PMID: 31100802 Free PMC article.
-
DNA-designed avian IgY antibodies: novel tools for research, diagnostics and therapy.J Clin Virol. 2005 Dec;34 Suppl 1:S70-4. doi: 10.1016/s1386-6532(05)80013-7. J Clin Virol. 2005. PMID: 16461227 Review.
-
Can Immunization of Hens Provide Oral-Based Therapeutics against COVID-19?Vaccines (Basel). 2020 Aug 28;8(3):486. doi: 10.3390/vaccines8030486. Vaccines (Basel). 2020. PMID: 32872186 Free PMC article. Review.
Cited by
-
Production and evaluation of rabies immunoglobulin extracted from chicken egg yolk.Biotechnol Rep (Amst). 2025 May 8;46:e00897. doi: 10.1016/j.btre.2025.e00897. eCollection 2025 Jun. Biotechnol Rep (Amst). 2025. PMID: 40487875 Free PMC article.
References
-
- Hatta H,Kim M andYamamoto T. A novel isolation method for hen egg yolk antibody, “IgY”. Agricultural and Biological Chemistry, 54: 2531–2535. 1990. - PubMed

