Acceleration of burn wound healing by micronized amniotic membrane seeded with umbilical cord-derived mesenchymal stem cells
- PMID: 37334186
- PMCID: PMC10276167
- DOI: 10.1016/j.mtbio.2023.100686
Acceleration of burn wound healing by micronized amniotic membrane seeded with umbilical cord-derived mesenchymal stem cells
Abstract
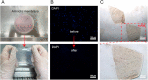
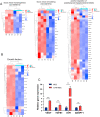
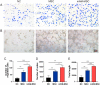
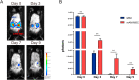
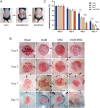
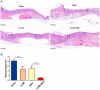
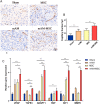
Umbilical cord-derived mesenchymal stem cells (UC-MSC) are promising candidates for wound healing. However, the low amplification efficiency of MSC in vitro and their low survival rates after transplantation have limited their medical application. In this study, we fabricated a micronized amniotic membrane (mAM) as a microcarrier to amplify MSC in vitro and used mAM and MSC (mAM-MSC) complexes to repair burn wounds. Results showed that MSC could live and proliferate on mAM in a 3D culture system, exhibiting higher cell activity than in 2D culture. Transcriptome sequencing of MSC showed that the expression of growth factor-related, angiogenesis-related, and wound healing-related genes was significantly upregulated in mAM-MSC compared to traditional 2D-cultured MSC, which was verified via RT-qPCR. Gene ontology (GO) analysis of differentially expressed genes (DEGs) showed significant enrichment of terms related to cell proliferation, angiogenesis, cytokine activity, and wound healing in mAM-MSC. In a burn wound model of C57BL/6J mice, topical application of mAM-MSC significantly accelerated wound healing compared to MSC injection alone and was accompanied by longer survival of MSC and greater neovascularization in the wound.
Keywords: Amniotic membrane; Burn wound healing; Microcarrier; Umbilical cord-derived mesenchymal stem cells.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Yao Y., Zhang A., Yuan C., Chen X., Liu Y. Recent trends on burn wound care: hydrogel dressings and scaffolds. Biomater. Sci. 2021;9(13):4523–4540. - PubMed
-
- M G.E.B., Dosh L., Haidar H., Gerges A., Baassiri S., Leone A., Rappa F., Jurjus A. Burns; 2022. Nerve Growth Factor and Burn Wound Healing: Update of Molecular Interactions with Skin Cells. - PubMed
-
- Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453(7193):314–321. - PubMed
-
- Singh V., Devgan L., Bhat S., Milner S.M. The pathogenesis of burn wound conversion. Ann. Plast. Surg. 2007;59(1):109–115. - PubMed
LinkOut - more resources
Full Text Sources

