Precise mapping of one classic and three novel GluRIIA mutants in Drosophila melanogaster
- PMID: 37334199
- PMCID: PMC10276266
- DOI: 10.17912/micropub.biology.000784
Precise mapping of one classic and three novel GluRIIA mutants in Drosophila melanogaster
Abstract
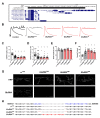
Mutation of the Drosophila melanogaster GluRIIA gene or pharmacological agents targeting it are commonly used to assess homeostatic synaptic function at the larval neuromuscular junction (NMJ). The commonly used mutation, GluRIIA SP16 , is a null allele created by a large and imprecise excision of a P-element which affects GluRIIA and multiple upstream genes. Here we mapped the exact bounds of the GluRIIA SP16 allele, refined a multiplex PCR strategy for positive identification of GluRIIA SP16 in homozygous or heterozygous backgrounds, and sequenced and characterized three new CRISPR-generated GluRIIA mutants. We found the three new GluRIIA alleles are apparent nulls that lack GluRIIA immunofluorescence signal at the 3 rd instar larval NMJ and are predicted to cause premature truncations at the genetic level. Further, these new mutants have similar electrophysiological outcomes as GluRIIA SP16 , including reduced miniature excitatory postsynaptic potential (mEPSP) amplitude and frequency compared to controls, and they express robust homeostatic compensation as evidenced by normal excitatory postsynaptic potential (EPSP) amplitude and elevated quantal content. These findings and new tools extend the capacity of the D. melanogaster NMJ for assessment of synaptic function.
Copyright: © 2023 by the authors.
Conflict of interest statement
The authors declare that there are no conflicts of interest present.
Figures
Similar articles
-
Versatile Endogenous Editing of GluRIIA in Drosophila melanogaster.Cells. 2024 Feb 10;13(4):323. doi: 10.3390/cells13040323. Cells. 2024. PMID: 38391936 Free PMC article.
-
Drosophila Mon1 and Rab7 interact to regulate glutamate receptor levels at the neuromuscular junction.Int J Dev Biol. 2020;64(4-5-6):289-297. doi: 10.1387/ijdb.190153ar. Int J Dev Biol. 2020. PMID: 32658990
-
Eliciting Presynaptic Homeostatic Potentiation at the Drosophila Larval Neuromuscular Junction.Cold Spring Harb Protoc. 2025 May 5;2025(5):pdb.prot108424. doi: 10.1101/pdb.prot108424. Cold Spring Harb Protoc. 2025. PMID: 38688541
-
Homeostatic plasticity at the Drosophila neuromuscular junction.Neuropharmacology. 2014 Mar;78:63-74. doi: 10.1016/j.neuropharm.2013.06.015. Epub 2013 Jun 24. Neuropharmacology. 2014. PMID: 23806804 Free PMC article. Review.
-
Different mechanisms of Ca2+ regulation that influence synaptic transmission: comparison between crayfish and Drosophila neuromuscular junctions.Synapse. 2009 Dec;63(12):1100-21. doi: 10.1002/syn.20695. Synapse. 2009. PMID: 19650116 Review.
Cited by
-
Distinct Bin/Amphiphysin/Rvs (BAR) family proteins may assemble on the same tubule to regulate membrane organization in vivo.Heliyon. 2024 Jun 26;10(13):e33672. doi: 10.1016/j.heliyon.2024.e33672. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39040266 Free PMC article.
-
Versatile Endogenous Editing of GluRIIA in Drosophila melanogaster.Cells. 2024 Feb 10;13(4):323. doi: 10.3390/cells13040323. Cells. 2024. PMID: 38391936 Free PMC article.
References
-
- Brusich Douglas J. Dual roles for an intracellular calcium-signaling pathway in regulating synaptic homeostasis and neuronal excitability. 2023 Mar 23; doi: 10.17077/etd.3eobkf2w. - DOI
-
- Brusich Douglas J., Spring Ashlyn M., James Thomas D., Yeates Catherine J., Helms Timothy H., Frank C. Andrew. Drosophila CaV2 channels harboring human migraine mutations cause synapse hyperexcitability that can be suppressed by inhibition of a Ca2+ store release pathway. PLOS Genetics. 2018 Aug 6;14(8):e1007577–e1007577. doi: 10.1371/journal.pgen.1007577. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
