A comprehensive history of motility and Archaellation in Archaea
- PMID: 37334237
- PMCID: PMC10117864
- DOI: 10.1093/femsmc/xtab002
A comprehensive history of motility and Archaellation in Archaea
Abstract
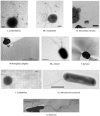
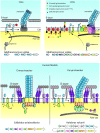
Each of the three Domains of life, Eukarya, Bacteria and Archaea, have swimming structures that were all originally called flagella, despite the fact that none were evolutionarily related to either of the other two. Surprisingly, this was true even in the two prokaryotic Domains of Bacteria and Archaea. Beginning in the 1980s, evidence gradually accumulated that convincingly demonstrated that the motility organelle in Archaea was unrelated to that found in Bacteria, but surprisingly shared significant similarities to type IV pili. This information culminated in the proposal, in 2012, that the 'archaeal flagellum' be assigned a new name, the archaellum. In this review, we provide a historical overview on archaella and motility research in Archaea, beginning with the first simple observations of motile extreme halophilic archaea a century ago up to state-of-the-art cryo-tomography of the archaellum motor complex and filament observed today. In addition to structural and biochemical data which revealed the archaellum to be a type IV pilus-like structure repurposed as a rotating nanomachine (Beeby et al. 2020), we also review the initial discoveries and subsequent advances using a wide variety of approaches to reveal: complex regulatory events that lead to the assembly of the archaellum filaments (archaellation); the roles of the various archaellum proteins; key post-translational modifications of the archaellum structural subunits; evolutionary relationships; functions of archaella other than motility and the biotechnological potential of this fascinating structure. The progress made in understanding the structure and assembly of the archaellum is highlighted by comparing early models to what is known today.
Keywords: archaea; assembly; motility; regulation; structural biology; surface structures.
© The Author(s) 2021. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
None declared.
Figures







References
-
- Abdul Halim MF, Pfeiffer F, Zou Jet al. Haloferax volcanii archaeosortase is required for motility, mating, and C-terminal processing of the S-layer glycoprotein. Mol Microbiol. 2013;88:1164–75. - PubMed
-
- Akinyemi T, Shao N, Whitman WB. Methanothermaceae. In Bergey's Manual of Systematics of Archaea and Bacteria. In: Trujillo ME, Dedysh S, DeVos P. et al. (eds.). 2021. 10.1002/9781118960608.fbm00098.pub2. - DOI
-
- Alam M, Oesterhelt D. Morphology, function and isolation of halobacterial flagella. J Mol Biol. 1984;176:459–75. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
