Changes and related factors of blood CCN1 levels in diabetic patients
- PMID: 37334311
- PMCID: PMC10273100
- DOI: 10.3389/fendo.2023.1131993
Changes and related factors of blood CCN1 levels in diabetic patients
Abstract
Objective: To study the differences in blood cellular communication network factor 1 (CCN1) levels between patients with diabetes mellitus (DM) and healthy individuals and to explore the relationship between CCN1 and diabetic retinopathy (DR).
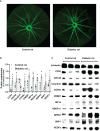
Methods: Plasma CCN1 levels were detected using ELISA in 50 healthy controls, 74 patients with diabetes without diabetic retinopathy (DM group), and 69 patients with diabetic retinopathy (DR group). Correlations between CCN1 levels and age, body mass index, mean arterial pressure, hemoglobin A1c, and other factors were analyzed. The relationship between CCN1 expression and DR was explored using logistic regression after adjusting for confounding factors. Blood mRNA sequencing analysis was performed for all subjects, and the molecular changes that may be related to CCN1 were explored. The retinal vasculature of streptozotocin-induced diabetic rats was examined using fundus fluorescein angiography; in addition, retinal protein expression was examined using western blotting.
Results: Plasma CCN1 levels in patients with DR were significantly higher than in the control and DM groups; however, no significant differences were observed between healthy controls and patients with DM. CCN1 levels negatively correlated with body mass index and positively correlated with the duration of diabetes and urea levels. It was observed that high (OR 4.72, 95% CI: 1.10-20.25) and very high (OR 8.54, 95% CI: 2.00-36.51) levels of CCN1 were risk factors for DR. Blood mRNA sequencing analysis revealed that CCN1-related pathways were significantly altered in the DR group. The expression of hypoxia-, oxidative stress-, and dephosphorylation-related proteins were elevated, while that of tight junction proteins were reduced in the retinas of diabetic rats.
Conclusion: Blood CCN1 levels are significantly elevated in patients with DR. High and very high levels of plasma CCN1 are risk factors for DR. Blood CCN1 level may be a potential biomarker for diagnosis of DR. The effects of CCN1 on DR may be related to hypoxia, oxidative stress, and dephosphorylation.
Keywords: CCN1/Cyr61; blood biomarker; blood-retinal barrier; diabetic Retinopathy; diabetic complications.
Copyright © 2023 Xiang, Chen, Qin, Lin, Xu, Lu and Zou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

