Dengue virus neutralizing antibody: a review of targets, cross-reactivity, and antibody-dependent enhancement
- PMID: 37334355
- PMCID: PMC10272415
- DOI: 10.3389/fimmu.2023.1200195
Dengue virus neutralizing antibody: a review of targets, cross-reactivity, and antibody-dependent enhancement
Abstract
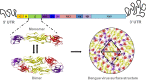
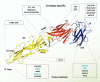
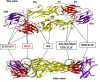
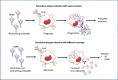
Dengue is the most common viral infection spread by mosquitoes, prevalent in tropical countries. The acute dengue virus (DENV) infection is a benign and primarily febrile illness. However, secondary infection with alternative serotypes can worsen the condition, leading to severe and potentially fatal dengue. The antibody raised by the vaccine or the primary infections are frequently cross-reactive; however, weakly neutralizing, and during subsequent infection, they may increase the odds of antibody-dependent enhancement (ADE). Despite that, many neutralizing antibodies have been identified against the DENV, which are thought to be useful in reducing dengue severity. Indeed, an antibody must be free from ADE for therapeutic application, as it is pretty common in dengue infection and escalates disease severity. Therefore, this review has described the critical characteristics of DENV and the potential immune targets in general. The primary emphasis is given to the envelope protein of DENV, where potential epitopes targeted for generating serotype-specific and cross-reactive antibodies have critically been described. In addition, a novel class of highly neutralizing antibodies targeted to the quaternary structure, similar to viral particles, has also been described. Lastly, we have discussed different aspects of the pathogenesis and ADE, which would provide significant insights into developing safe and effective antibody therapeutics and equivalent protein subunit vaccines.
Keywords: antibody engineering; antibody-dependent enhancement; cross-reactivity; dengue virus; neutralizing antibodies; subunit vaccine.
Copyright © 2023 Sarker, Dhama and Gupta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

