Host-mediated beneficial effects of phytochemicals for prevention of avian coccidiosis
- PMID: 37334385
- PMCID: PMC10272459
- DOI: 10.3389/fimmu.2023.1145367
Host-mediated beneficial effects of phytochemicals for prevention of avian coccidiosis
Abstract
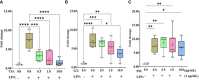
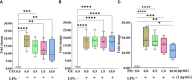
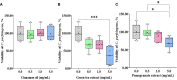
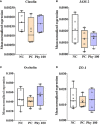
Both in vitro and in vivo studies were conducted to evaluate the beneficial effects of green tea extract (GT), cinnamon oil (CO), and pomegranate extract (PO) on avian coccidiosis. In experiment (EXP) 1, an in vitro culture system was used to investigate the individual effects of GT, CO, and PO on the proinflammatory cytokine response and integrity of tight junction (TJ) in chicken intestinal epithelial cells (IEC), on the differentiation of quail muscle cells and primary chicken embryonic muscle cells, and anticoccidial and antibacterial activities against Eimeria tenella sporozoites and Clostridium perfringens bacteria, respectively. In EXP 2 and 3, in vivo trials were carried out to study the dose-dependent effect of blended phytochemicals (GT, CO, PO) on coccidiosis in broiler chickens infected with E. maxima. For EXP 2, one hundred male broiler chickens (0-day-old) were allocated into the following five treatment groups: Control group for non-infected chickens (NC), Basal diet group for E. maxima-infected chickens (PC), PC group supplemented with phytochemicals at 50 (Phy 50), 100 (Phy 100), and 200 (Phy 200) mg/kg feed diets for E. maxima-infected chickens. For EXP 3, one hundred twenty male broiler chickens (0-day-old) were allocated into the following six treatment groups: NC, PC, PC supplemented with phytochemicals at 10 (Phy 10), 20 (Phy 20), 30 (Phy 30), and 100 (Phy 100) mg/kg feed for E. maxima-infected chickens. Body weights (BW) were measured on days 0, 7, 14, 20, and 22, and jejunum samples were used to measure cytokine, TJ protein, and antioxidant enzyme responses at 8 days post-infection (dpi). Fecal samples for oocyst enumeration were collected from 6 to 8 dpi. In vitro, CO and PO reduced LPS-induced IL-1β and IL-8 in IEC, respectively, and GT enhanced the gene expression of occludin in IEC. PO at 1.0 and 5.0 mg/mL exerted antimicrobial effect against E. tenella sporozoites and C. perfringens bacteria, respectively. In vivo, chickens fed a diet supplemented with phytochemicals showed enhanced BW, reduced oocyst shedding, and decreased proinflammatory cytokines following E. maxima challenge. In conclusion, the combination of GT, CO, and PO in the diet of broiler chickens infected with E. maxima induced enhanced host disease resistance including innate immunity and gut health, which contributed to improved growth and reduced disease responses. These findings provide scientific support for the development of a novel phytogenic feed additive formula that enhances the growth and intestinal health of broiler chickens infected with coccidiosis.
Keywords: Eimeria maxima; alternatives to antibiotics; broiler chicken; coccidiosis; growth; gut health; intestinal immunity; phytochemicals.
Copyright © 2023 Park, Nam, Wickramasuriya, Lee, Wall, Ravichandran and Lillehoj.
Conflict of interest statement
Authors EW and SR were employed by the company AVT natural. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Phytochemicals act holistically to enhance host defenses during poultry coccidiosis.Poult Sci. 2025 May;104(5):105042. doi: 10.1016/j.psj.2025.105042. Epub 2025 Mar 15. Poult Sci. 2025. PMID: 40120241 Free PMC article.
-
The effect of gut microbiota-derived carnosine on mucosal integrity and immunity in broiler chickens challenged with Eimeria maxima.Poult Sci. 2024 Aug;103(8):103837. doi: 10.1016/j.psj.2024.103837. Epub 2024 May 9. Poult Sci. 2024. PMID: 38848630 Free PMC article.
-
Gut Microbiota-Derived Indole-3-Carboxylate Influences Mucosal Integrity and Immunity Through the Activation of the Aryl Hydrocarbon Receptors and Nutrient Transporters in Broiler Chickens Challenged With Eimeria maxima.Front Immunol. 2022 Jun 23;13:867754. doi: 10.3389/fimmu.2022.867754. eCollection 2022. Front Immunol. 2022. PMID: 35812452 Free PMC article.
-
Phytochemical control of poultry coccidiosis: a review.Poult Sci. 2022 Jan;101(1):101542. doi: 10.1016/j.psj.2021.101542. Epub 2021 Oct 14. Poult Sci. 2022. PMID: 34871985 Free PMC article. Review.
-
Response of different infection models in broiler chickens against supplemental Organic acid - A review.Microb Pathog. 2025 Jul;204:107527. doi: 10.1016/j.micpath.2025.107527. Epub 2025 Apr 2. Microb Pathog. 2025. PMID: 40185170 Review.
Cited by
-
Effect of β-Alanine Metabolite on Gut Integrity and Immunity in Commercial Broiler Chickens Infected with Eimeria maxima.Animals (Basel). 2024 Sep 3;14(17):2558. doi: 10.3390/ani14172558. Animals (Basel). 2024. PMID: 39272343 Free PMC article.
-
Molecular responses to clove and oregano essential oils are associated with reduced inflammation and improved gut barrier function in broiler chickens.Poult Sci. 2025 Feb;104(2):104713. doi: 10.1016/j.psj.2024.104713. Epub 2024 Dec 20. Poult Sci. 2025. PMID: 39721262 Free PMC article.
-
Comparison of the effect of anticoccidial drug, probiotic, synbiotic, phytochemicals and vaccine in prevention and control of coccidiosis in broiler chickens challenged with Eimeria spp.Poult Sci. 2024 Dec;103(12):104357. doi: 10.1016/j.psj.2024.104357. Epub 2024 Sep 25. Poult Sci. 2024. PMID: 39426225 Free PMC article.
-
Effects of Anticoccidial Vaccination and Taraxacum officinale Extract on the Growth Performance, Biochemical Parameters, Immunity, and Intestinal Morphology of Eimeria-Challenged Chickens.Life (Basel). 2023 Sep 17;13(9):1927. doi: 10.3390/life13091927. Life (Basel). 2023. PMID: 37763330 Free PMC article.
-
Phytochemicals act holistically to enhance host defenses during poultry coccidiosis.Poult Sci. 2025 May;104(5):105042. doi: 10.1016/j.psj.2025.105042. Epub 2025 Mar 15. Poult Sci. 2025. PMID: 40120241 Free PMC article.
References
-
- Gadde U, Oh ST, Lee YS, Davis E, Zimmerman N, Rehberger T, et al. . The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics Antimicrob Proteins (2017) 9:397–405. doi: 10.1007/s12602-017-9275-9 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

