Comparative effectiveness of JAK inhibitors and biologic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis
- PMID: 37334513
- PMCID: PMC10338257
- DOI: 10.3904/kjim.2022.369
Comparative effectiveness of JAK inhibitors and biologic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis
Abstract
Background/aims: We aimed to compare the effectiveness and safety of Janus kinase inhibitors (JAKi) vs. biologic disease- modifying antirheumatic drugs (bDMARD) in Korean patients with rheumatoid arthritis (RA) who had an inadequate response to conventional synthetic DMARDs.
Methods: A quasi-experimental, multi-center, prospective, non-randomized study was conducted to compare response rates between JAKi and bDMARDs in patients with RA naïve to targeted therapy. An interim analysis was performed to estimate the proportion of patients achieving low disease activity (LDA) based on disease activity score (DAS)-28- erythroid sedimentation rate (ESR) (DAS28-ESR) at 24 weeks after treatment initiation and to evaluate the development of adverse events (AEs).
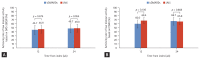
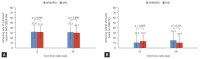
Results: Among 506 patients enrolled from 17 institutions between April 2020 and August 2022, 346 (196 JAKi group and 150 bDMARD group) were included in the analysis. After 24 weeks of treatment, 49.0% of JAKi users and 48.7% of bDMARD users achieved LDA (p = 0.954). DAS28-ESR remission rates were also comparable between JAKi and bDMARD users (30.1% and 31.3%, respectively; p = 0.806). The frequency of AEs reported in the JAKi group was numerically higher than that in the bDMARDs group, but the frequencies of serious and severe AEs were comparable between the groups.
Conclusion: Our interim findings reveal JAKi have comparable effectiveness and safety to bDMARDs at 24 weeks after treatment initiation.
Keywords: Comparative effectiveness research; Janus kinase inhibitor; Rheumatoid arthritis; Safety; Tumor necrosis factor inhibitors.
Conflict of interest statement
The authors disclose no conflicts.
Figures


Similar articles
-
Real-world comparative effectiveness study of Janus kinase inhibitors compared to biologic disease-modifying antirheumatic drugs in Korean patients with rheumatoid arthritis.Semin Arthritis Rheum. 2025 Aug;73:152720. doi: 10.1016/j.semarthrit.2025.152720. Epub 2025 Apr 14. Semin Arthritis Rheum. 2025. PMID: 40245585
-
A retrospective study of efficacy of tofacitinib combined with bDMARDs in the treatment of rheumatoid arthritis patients with inadequate response to bDMARDs.Int J Rheum Dis. 2024 Sep;27(9):e15311. doi: 10.1111/1756-185X.15311. Int J Rheum Dis. 2024. PMID: 39198040
-
Drug survival and change of disease activity using a second janus kinase inhibitor in patients with difficult-to-treat rheumatoid arthritis who failed to a janus kinase inhibitor and subsequent biologics.Adv Rheumatol. 2024 Apr 15;64(1):26. doi: 10.1186/s42358-024-00368-w. Adv Rheumatol. 2024. PMID: 38622706
-
How to Use Janus Kinase Inhibitors in the Treatment of Rheumatoid Arthritis? A Clinical Assessment of Risks and Benefits.Curr Rheumatol Rep. 2023 Dec;25(12):295-306. doi: 10.1007/s11926-023-01122-9. Epub 2023 Dec 16. Curr Rheumatol Rep. 2023. PMID: 38102522 Review.
-
Current jakinibs for the treatment of rheumatoid arthritis: a systematic review.Inflammopharmacology. 2021 Jun;29(3):595-615. doi: 10.1007/s10787-021-00822-x. Epub 2021 May 27. Inflammopharmacology. 2021. PMID: 34046798
Cited by
-
Are JAKis more effective among elderly patients with RA, smokers and those with higher cardiovascular risk? A comparative effectiveness study of b/tsDMARDs in Sweden.RMD Open. 2023 Dec 26;9(4):e003648. doi: 10.1136/rmdopen-2023-003648. RMD Open. 2023. PMID: 38151264 Free PMC article.
-
Impact of age and cardiovascular risk factors on the incidence of adverse events in patients with rheumatoid arthritis treated with Janus Kinase inhibitors: data from a real-life multicentric cohort.Clin Exp Med. 2024 Mar 30;24(1):62. doi: 10.1007/s10238-024-01325-z. Clin Exp Med. 2024. PMID: 38554250 Free PMC article.
References
-
- Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. - PubMed
-
- Burmester GR, Blanco R, Charles-Schoeman C, et al. ORAL Step investigators Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
