Improving heart failure outcomes with sodium-glucose cotransporter 2 inhibitors in different patient groups
- PMID: 37334518
- PMCID: PMC10946463
- DOI: 10.1111/dom.15171
Improving heart failure outcomes with sodium-glucose cotransporter 2 inhibitors in different patient groups
Abstract
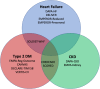
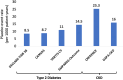
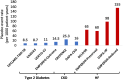
Sodium-glucose cotransporter 2 inhibitors (SGLT-2is) were originally developed for the treatment of hyperglycaemia in type 2 diabetes. Because of regulatory requirements to show the safety of this new class of drugs, a large randomized cardiovascular (CV) outcomes trial was completed but this showed that instead of having a neutral effect on heart failure (HF) outcomes, that these drugs could reduce HF outcomes in this population. Subsequent trials with SGLT-2is have shown that HF hospitalizations are reduced by 30% and CV death or HF hospitalization by 21% in patients with type 2 diabetes. These findings have extended to patients with HF with reduced and mildly reduced or preserved ejection fraction in whom further HF hospitalizations are reduced by 28% and CV death or HF hospitalizations reduced by 23%, and that it is becoming a central therapy for the treatment of HF. Moreover, the benefit in patients with HF is observed regardless of the presence or absence of type 2 diabetes. Similarly, in patients with chronic kidney disease and albuminuria, with and without type 2 diabetes, the benefit of SGLT-2is is clearly seen with a 44% reduction in HF hospitalization and 25% reduction in CV death or HF hospitalization. These trials support the use of SGLT-2is in improving HF outcomes in a broad range of patients, from those with type 2 diabetes, chronic kidney disease and those with pre-existing HF regardless of ejection fraction.
Keywords: SGLT-2 inhibitor; cardiovascular disease; drug development; drug mechanism; heart failure.
© 2023 The Author. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
PSJ reports speakers' fees from AstraZeneca, Novartis, Alkem Metabolics, ProAdWise Communications, Sun Pharmaceuticals and Intas Pharma; advisory board fees from AstraZeneca, Boehringer Ingelheim and Novartis; research funding from AstraZeneca, Boehringer Ingelheim, Analog Devices Inc. and Roche Diagnostics. PSJ employer, the University of Glasgow, has been remunerated for clinical trial work from AstraZeneca, Bayer AG, Novartis and Novo Nordisk. Director, Global Clinical Trial Partners (GCTP).
Figures

References
-
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. - PubMed
-
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. - PubMed
-
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347‐357. - PubMed
-
- Cannon CP, Pratley R, Dagogo‐Jack S, et al. Cardiovascular outcomes with Ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425‐1435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

