Automated treatment planning for proton pencil beam scanning using deep learning dose prediction and dose-mimicking optimization
- PMID: 37334746
- PMCID: PMC10562035
- DOI: 10.1002/acm2.14065
Automated treatment planning for proton pencil beam scanning using deep learning dose prediction and dose-mimicking optimization
Abstract
Purpose: The purpose of this study is to investigate the use of a deep learning architecture for automated treatment planning for proton pencil beam scanning (PBS).
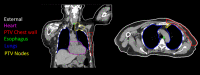
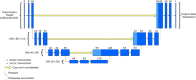
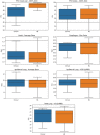
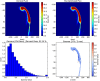
Methods: A 3-dimensional (3D) U-Net model has been implemented in a commercial treatment planning system (TPS) that uses contoured regions of interest (ROI) binary masks as model inputs with a predicted dose distribution as the model output. Predicted dose distributions were converted to deliverable PBS treatment plans using a voxel-wise robust dose mimicking optimization algorithm. This model was leveraged to generate machine learning (ML) optimized plans for patients receiving proton PBS irradiation of the chest wall. Model training was carried out on a retrospective set of 48 previously-treated chest wall patient treatment plans. Model evaluation was carried out by generating ML-optimized plans on a hold-out set of 12 contoured chest wall patient CT datasets from previously treated patients. Clinical goal criteria and gamma analysis were used to compare dose distributions of the ML-optimized plans against the clinically approved plans across the test patients.
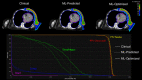
Results: Statistical analysis of mean clinical goal criteria indicates that compared to the clinical plans, the ML optimization workflow generated robust plans with similar dose to the heart, lungs, and esophagus while achieving superior dosimetric coverage to the PTV chest wall (clinical mean V95 = 97.6% vs. ML mean V95 = 99.1%, p < 0.001) across the 12 test patients.
Conclusions: ML-based automated treatment plan optimization using the 3D U-Net model can generate treatment plans of similar clinical quality compared to human-driven optimization.
Keywords: deep learning; proton therapy.
© 2023 The Authors. Journal of Applied Clinical Medical Physics published by Wiley Periodicals, LLC on behalf of The American Association of Physicists in Medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Goodfellow IJ, Pouget–Abadie J, Mirza M, et al. Generative adversarial networks. Commun Acm. 2020;63(11):139–144.
-
- Mahmood R, Babier A, McNiven A, Diamant A, Chan TC. Automated Treatment Planning in Radiation Therapy Using Generative Adversarial Networks. PMLR; 2018:484‐499.
-
- McIntosh C, Welch M, McNiven A, Jaffray DA, Purdie TG. Fully automated treatment planning for head and neck radiotherapy using a voxel‐based dose prediction and dose mimicking method. Phys Med Biol. 2017;62(15):5926. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

