Extracellular matrix composition alters endothelial force transmission
- PMID: 37335028
- PMCID: PMC10393341
- DOI: 10.1152/ajpcell.00106.2023
Extracellular matrix composition alters endothelial force transmission
Abstract
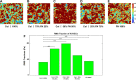
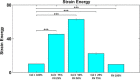
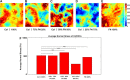
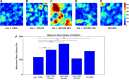
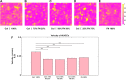
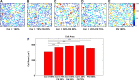
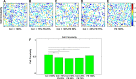
Extracellular matrix (ECM) composition is important in a host of pathophysiological processes such as angiogenesis, atherosclerosis, and diabetes, and during each of these processes ECM composition has been reported to change over time. However, the impact ECM composition has on the ability of endothelium to respond mechanically is currently unknown. Therefore, in this study, we seeded human umbilical vein endothelial cells (HUVECs) onto soft hydrogels coated with an ECM concentration of 0.1 mg/mL at the following collagen I (Col-I) and fibronectin (FN) ratios: 100% Col-I, 75% Col-I-25% FN, 50% Col-I-50% FN, 25% Col-I-75% FN, and 100% FN. We subsequently measured tractions, intercellular stresses, strain energy, cell morphology, and cell velocity. Our results revealed that tractions and strain energy are maximal at 50% Col-I-50% FN and minimal at 100% Col-I and 100% FN. Intercellular stress response was maximal on 50% Col-I-50% FN and minimal on 25% Col-I-75% FN. Cell area and cell circularity displayed a divergent relationship for different Col-I and FN ratios. We believe that these results will be of great importance to the cardiovascular field, biomedical field, and cell mechanics.NEW & NOTEWORTHY The endothelium constitutes the innermost layer of all blood vessels and plays an important role in vascular physiology and pathology. During certain vascular diseases, the extracellular matrix has been suggested to transition from a collagen-rich matrix to a fibronectin-rich matrix. In this study, we demonstrate the impact various collagen and fibronectin ratios have on endothelial biomechanical and morphological response.
Keywords: extracellular matrix; fibronectin; intercellular stresses; traction force microscopy; type 1 collagen.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Update of
-
Extracellular Matrix Composition Alters Endothelial Force Transmission.Res Sq [Preprint]. 2023 Jan 27:rs.3.rs-2499973. doi: 10.21203/rs.3.rs-2499973/v1. Res Sq. 2023. Update in: Am J Physiol Cell Physiol. 2023 Jul 1;325(1):C314-C323. doi: 10.1152/ajpcell.00106.2023. PMID: 36747754 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

