Amyloids and brain cancer: molecular linkages and crossovers
- PMID: 37335084
- PMCID: PMC10548166
- DOI: 10.1042/BSR20230489
Amyloids and brain cancer: molecular linkages and crossovers
Abstract
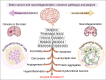
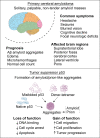
Amyloids are high-order proteinaceous formations deposited in both intra- and extracellular spaces. These aggregates have tendencies to deregulate cellular physiology in multiple ways; for example, altered metabolism, mitochondrial dysfunctions, immune modulation, etc. When amyloids are formed in brain tissues, the endpoint often is death of neurons. However, interesting but least understood is a close connection of amyloids with another set of conditions in which brain cells proliferate at an extraordinary rate and form tumor inside brain. Glioblastoma is one such condition. Increasing number of evidence indicate a possible link between amyloid formation and depositions in brain tumors. Several proteins associated with cell cycle regulation and apoptotic pathways themselves have shown to possess high tendencies to form amyloids. Tumor suppressor protein p53 is one prominent example that mutate, oligomerize and form amyloids leading to loss- or gain-of-functions and cause increased cell proliferation and malignancies. In this review article, we present available examples, genetic links and common pathways that indicate that possibly the two distantly placed pathways: amyloid formation and developing cancers in the brain have similarities and are mechanistically intertwined together.
Keywords: Cancer; aging; amyloid; glioma; neurodegeneration; proteostasis.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
-
- Costa D.C., de Oliveira G.A., Cino E.A., Soares I.N., Rangel L.P. and Silva J.L. (2016) Aggregation and prion-like properties of misfolded tumor suppressors: is cancer a prion disease? Cold Spring Harb. Perspect. Biol. 8, 1–21Epub 2016/08/24. PubMed Central PMCID: PMCPMC5046694 10.1101/cshperspect.a023614 - DOI - PMC - PubMed
-
- Zayas-Santiago A., Martinez-Montemayor M.M., Colon-Vazquez J., Ortiz-Soto G., Cirino-Simonet J.G. and Inyushin M. (2022) Accumulation of amyloid beta (Abeta) and amyloid precursor protein (APP) in tumors formed by a mouse xenograft model of inflammatory breast cancer. FEBS Open Bio 12, 95–105, 10.1002/2211-5463.13308 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

