A Lightweight Drive Implant for Chronic Tetrode Recordings in Juvenile Mice
- PMID: 37335110
- PMCID: PMC10903788
- DOI: 10.3791/65228
A Lightweight Drive Implant for Chronic Tetrode Recordings in Juvenile Mice
Abstract
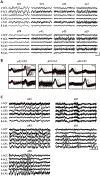
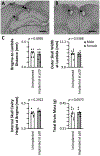
In vivo electrophysiology provides unparalleled insight into the sub-second-level circuit dynamics of the intact brain and represents a method of particular importance for studying mouse models of human neuropsychiatric disorders. However, such methods often require large cranial implants, which cannot be used in mice at early developmental time points. As such, virtually no studies of in vivo physiology have been performed in freely behaving infant or juvenile mice, despite the fact that a better understanding of neurological development in this critical window would likely provide unique insights into age-dependent developmental disorders such as autism or schizophrenia. Here, a micro-drive design, surgical implantation procedure, and post-surgery recovery strategy are described that allow for chronic field and single-unit recordings from multiple brain regions simultaneously in mice as they age from postnatal day 20 (p20) to postnatal day 60 (p60) and beyond, a time window roughly corresponding to the human ages of 2 years old through to adulthood. The number of recording electrodes and final recording sites can be easily modified and expanded, thus allowing flexible experimental control of the in vivo monitoring of behavior- or disease-relevant brain regions across development.
Figures



Update of
-
A novel, lightweight drive implant for chronic tetrode recordings in juvenile mice.bioRxiv [Preprint]. 2023 Jan 5:2023.01.04.522760. doi: 10.1101/2023.01.04.522760. bioRxiv. 2023. Update in: J Vis Exp. 2023 Jun 2;(196). doi: 10.3791/65228. PMID: 36711560 Free PMC article. Updated. Preprint.
Similar articles
-
A novel, lightweight drive implant for chronic tetrode recordings in juvenile mice.bioRxiv [Preprint]. 2023 Jan 5:2023.01.04.522760. doi: 10.1101/2023.01.04.522760. bioRxiv. 2023. Update in: J Vis Exp. 2023 Jun 2;(196). doi: 10.3791/65228. PMID: 36711560 Free PMC article. Updated. Preprint.
-
An adaptable, reusable, and light implant for chronic Neuropixels probes.Elife. 2025 Feb 18;13:RP98522. doi: 10.7554/eLife.98522. Elife. 2025. PMID: 39964835 Free PMC article.
-
The DREAM Implant: A Lightweight, Modular, and Cost-Effective Implant System for Chronic Electrophysiology in Head-Fixed and Freely Behaving Mice.J Vis Exp. 2024 Jul 26;(209). doi: 10.3791/66867. J Vis Exp. 2024. PMID: 39141557
-
Intracellular recording in behaving animals.Curr Opin Neurobiol. 2012 Feb;22(1):34-44. doi: 10.1016/j.conb.2011.10.013. Epub 2011 Nov 3. Curr Opin Neurobiol. 2012. PMID: 22054814 Free PMC article. Review.
-
Studying sensorimotor processing with physiology in behaving Drosophila.Int Rev Neurobiol. 2011;99:169-89. doi: 10.1016/B978-0-12-387003-2.00007-0. Int Rev Neurobiol. 2011. PMID: 21906540 Review.
References
-
- Volk L, Chiu S-L, Sharma K, Huganir RL Glutamate synapses in human cognitive disorders. Annual Review of Neuroscience. 38, 127–149 (2015). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
