Targeting CaMKK2 Inhibits Actin Cytoskeletal Assembly to Suppress Cancer Metastasis
- PMID: 37335130
- PMCID: PMC10472110
- DOI: 10.1158/0008-5472.CAN-22-1622
Targeting CaMKK2 Inhibits Actin Cytoskeletal Assembly to Suppress Cancer Metastasis
Abstract
Triple-negative breast cancers (TNBC) tend to become invasive and metastatic at early stages in their development. Despite some treatment successes in early-stage localized TNBC, the rate of distant recurrence remains high, and long-term survival outcomes remain poor. In a search for new therapeutic targets for this disease, we observed that elevated expression of the serine/threonine kinase calcium/calmodulin (CaM)-dependent protein kinase kinase 2 (CaMKK2) is highly correlated with tumor invasiveness. In validation studies, genetic disruption of CaMKK2 expression or inhibition of its activity with small molecule inhibitors disrupted spontaneous metastatic outgrowth from primary tumors in murine xenograft models of TNBC. High-grade serous ovarian cancer (HGSOC), a high-risk, poor prognosis ovarian cancer subtype, shares many features with TNBC, and CaMKK2 inhibition effectively blocked metastatic progression in a validated xenograft model of this disease. Mechanistically, CaMKK2 increased the expression of the phosphodiesterase PDE1A, which hydrolyzed cyclic guanosine monophosphate (cGMP) to decrease the cGMP-dependent activity of protein kinase G1 (PKG1). Inhibition of PKG1 resulted in decreased phosphorylation of vasodilator-stimulated phosphoprotein (VASP), which in its hypophosphorylated state binds to and regulates F-actin assembly to facilitate cell movement. Together, these findings establish a targetable CaMKK2-PDE1A-PKG1-VASP signaling pathway that controls cancer cell motility and metastasis by impacting the actin cytoskeleton. Furthermore, it identifies CaMKK2 as a potential therapeutic target that can be exploited to restrict tumor invasiveness in patients diagnosed with early-stage TNBC or localized HGSOC.
Significance: CaMKK2 regulates actin cytoskeletal dynamics to promote tumor invasiveness and can be inhibited to suppress metastasis of breast and ovarian cancer, indicating CaMKK2 inhibition as a therapeutic strategy to arrest disease progression.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures
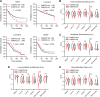

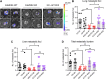
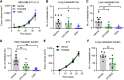
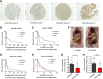
![Figure 5. Depletion of CaMKK2 leads to increased phosphorylation of VASP at the Serine 239 residue, which leads to impaired cytoskeletal assembly and cell motility. A, Representative images showing impaired cytoskeletal assembly in CaMKK2-KO cells. Visualization of F-actin by phalloidin staining in CaMKK2-WT (Ctrl #1) and CaMKK2-KO (KK2-KO #1) cells revealed almost complete loss of ventral stress fibers in cells lacking CaMKK2 expression. Yellow arrows, dorsal stress fibers; white arrows, ventral stress fibers; yellow bracket, transverse arcs; green arrowhead, leading edge; yellow arrowhead, contractile rear of the cell. Scale bar, 10 μm. B, Representative blot showing increased phosphorylation of VASP at Serine 239 in CaMMK2 KO cell clones. C, Representative blot showing VASP phosphorylation at Serine 239 is enhanced in CaMKK2-KO cells in response to ionomycin (1 μmol). Cells were treated with either DMSO or ionomycin (1 μmol/L) with CaCL2 (1 mmol/L) for varying time periods (as indicated). D and E, Inhibition of CaMKK2 (STO-609, 10 μmol) increases phosphorylation of VASP [especially with ionomycin (1 μmol; 2 hours)] in metastatic MDA-MB-231-4175 cells (D) and BT-20 cells (E). DMSO or STO-609–treated cells were exposed to either DMSO or ionomycin (1 μmol/L) with CaCL2 (1 mmol/L) for 2 hours before immunoblotting. F and G, CaMKK2 inhibition decreases migration of MDA-MB-231-4175 cells (F) and BT20 cells (G) in vitro. DMSO or STO-609–treated cells were exposed to either DMSO or ionomycin with CaCL2 (see D and E) before migration assays were performed. Data are plotted as mean ± SEM; n = 4–5 random fields measurements from four individual experiments done in duplicate. *, P < 0.05; **, P < 0.01; ***, P < 0.001. P values were calculated using unpaired Student t test.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/ac7d/10472110/3ff937a0fc39/2889fig5.gif)


References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. - PubMed
-
- Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023–75. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

