Antimicrobial Transformation Products in the Aquatic Environment: Global Occurrence, Ecotoxicological Risks, and Potential of Antibiotic Resistance
- PMID: 37335844
- PMCID: PMC10324322
- DOI: 10.1021/acs.est.2c09854
Antimicrobial Transformation Products in the Aquatic Environment: Global Occurrence, Ecotoxicological Risks, and Potential of Antibiotic Resistance
Abstract
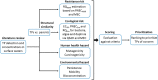
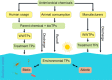
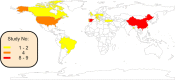
The global spread of antimicrobial resistance (AMR) is concerning for the health of humans, animals, and the environment in a One Health perspective. Assessments of AMR and associated environmental hazards mostly focus on antimicrobial parent compounds, while largely overlooking their transformation products (TPs). This review lists antimicrobial TPs identified in surface water environments and examines their potential for AMR promotion, ecological risk, as well as human health and environmental hazards using in silico models. Our review also summarizes the key transformation compartments of TPs, related pathways for TPs reaching surface waters and methodologies for studying the fate of TPs. The 56 antimicrobial TPs covered by the review were prioritized via scoring and ranking of various risk and hazard parameters. Most data on occurrences to date have been reported in Europe, while little is known about antibiotic TPs in Africa, Central and South America, Asia, and Oceania. Occurrence data on antiviral TPs and other antibacterial TPs are even scarcer. We propose evaluation of structural similarity between parent compounds and TPs for TP risk assessment. We predicted a risk of AMR for 13 TPs, especially TPs of tetracyclines and macrolides. We estimated the ecotoxicological effect concentrations of TPs from the experimental effect data of the parent chemical for bacteria, algae and water fleas, scaled by potency differences predicted by quantitative structure-activity relationships (QSARs) for baseline toxicity and a scaling factor for structural similarity. Inclusion of TPs in mixtures with their parent increased the ecological risk quotient over the threshold of one for 7 of the 24 antimicrobials included in this analysis, while only one parent had a risk quotient above one. Thirteen TPs, from which 6 were macrolide TPs, posed a risk to at least one of the three tested species. There were 12/21 TPs identified that are likely to exhibit a similar or higher level of mutagenicity/carcinogenicity, respectively, than their parent compound, with tetracycline TPs often showing increased mutagenicity. Most TPs with increased carcinogenicity belonged to sulfonamides. Most of the TPs were predicted to be mobile but not bioaccumulative, and 14 were predicted to be persistent. The six highest-priority TPs originated from the tetracycline antibiotic family and antivirals. This review, and in particular our ranking of antimicrobial TPs of concern, can support authorities in planning related intervention strategies and source mitigation of antimicrobials toward a sustainable future.
Keywords: antimicrobial resistance; chemical prioritization; degradation products; environmental analysis; metabolites; micropollutants; risk assessment; surface water.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

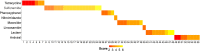
References
-
- Murray C. J.; Ikuta K. S.; Sharara F.; Swetschinski L.; Robles Aguilar G.; Gray A.; Han C.; Bisignano C.; Rao P.; Wool E.; Johnson S. C.; Browne A. J.; Chipeta M. G.; Fell F.; Hackett S.; Haines-Woodhouse G.; Kashef Hamadani B. H.; Kumaran E. A. P.; McManigal B.; Agarwal R.; Akech S.; Albertson S.; Amuasi J.; Andrews J.; Aravkin A.; Ashley E.; Bailey F.; Baker S.; Basnyat B.; Bekker A.; Bender R.; Bethou A.; Bielicki J.; Boonkasidecha S.; Bukosia J.; Carvalheiro C.; Castañeda-Orjuela C.; Chansamouth V.; Chaurasia S.; Chiurchiù S.; Chowdhury F.; Cook A. J.; Cooper B.; Cressey T. R.; Criollo-Mora E.; Cunningham M.; Darboe S.; Day N. P. J.; De Luca M.; Dokova K.; Dramowski A.; Dunachie S. J.; Eckmanns T.; Eibach D.; Emami A.; Feasey N.; Fisher-Pearson N.; Forrest K.; Garrett D.; Gastmeier P.; Giref A. Z.; Greer R. C.; Gupta V.; Haller S.; Haselbeck A.; Hay S. I.; Holm M.; Hopkins S.; Iregbu K. C.; Jacobs J.; Jarovsky D.; Javanmardi F.; Khorana M.; Kissoon N.; Kobeissi E.; Kostyanev T.; Krapp F.; Krumkamp R.; Kumar A.; Kyu H. H.; Lim C.; Limmathurotsakul D.; Loftus M. J.; Lunn M.; Ma J.; Mturi N.; Munera-Huertas T.; Musicha P.; Mussi-Pinhata M. M.; Nakamura T.; Nanavati R.; Nangia S.; Newton P.; Ngoun C.; Novotney A.; Nwakanma D.; Obiero C. W.; Olivas-Martinez A.; Olliaro P.; Ooko E.; Ortiz-Brizuela E.; Peleg A. Y.; Perrone C.; Plakkal N.; Ponce-de-Leon A.; Raad M.; Ramdin T.; Riddell A.; Roberts T.; Robotham J. V.; Roca A.; Rudd K. E.; Russell N.; Schnall J.; Scott J. A. G.; Shivamallappa M.; Sifuentes-Osornio J.; Steenkeste N.; Stewardson A. J.; Stoeva T.; Tasak N.; Thaiprakong A.; Thwaites G.; Turner C.; Turner P.; van Doorn H. R.; Velaphi S.; Vongpradith A.; Vu H.; Walsh T.; Waner S.; Wangrangsimakul T.; Wozniak T.; Zheng P.; Sartorius B.; Lopez A. D.; Stergachis A.; Moore C.; Dolecek C.; Naghavi M.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399 (10325), 629–655. 10.1016/S0140-6736(21)02724-0. - DOI - PMC - PubMed
-
- World Health Organization . Ten health issues WHO will tackle this year. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-... (accessed 2020–11–28).
-
- Browne A. J.; Chipeta M. G.; Haines-Woodhouse G.; Kumaran E. P. A.; Hamadani B. H. K.; Zaraa S.; Henry N. J.; Deshpande A.; Reiner R. C.; Day N. P. J.; Lopez A. D.; Dunachie S.; Moore C. E.; Stergachis A.; Hay S. I.; Dolecek C. Global Antibiotic Consumption and Usage in Humans, 2000–18: A Spatial Modelling Study. Lancet Planetary Health 2021, 5 (12), e893–e904. 10.1016/S2542-5196(21)00280-1. - DOI - PMC - PubMed
-
- Klein E. Y.; Van Boeckel T. P.; Martinez E. M.; Pant S.; Gandra S.; Levin S. A.; Goossens H.; Laxminarayan R. Global Increase and Geographic Convergence in Antibiotic Consumption between 2000 and 2015. Proc. Natl. Acad. Sci. U. S. A. 2018, 115 (15), E3463–E3470. 10.1073/pnas.1717295115. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

