Evaluating Survival After Hospitalization Due to Immune-Related Adverse Events From Checkpoint Inhibitors
- PMID: 37335906
- PMCID: PMC10546826
- DOI: 10.1093/oncolo/oyad135
Evaluating Survival After Hospitalization Due to Immune-Related Adverse Events From Checkpoint Inhibitors
Abstract
Background: As immune checkpoint inhibitors (CPI) are increasingly approved for cancer treatment, hospitalizations related to severe immune-related adverse events (irAE) will increase. Here, we identify patients hospitalized due to irAEs and describe survival outcomes across irAE, CPI, and cancer type.
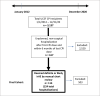
Methods: We identified patients hospitalized at our institution from January 2012 to December 2020 due to irAEs. Survival was analyzed using Kaplan-Meier survival curves with log-rank tests.
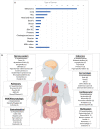
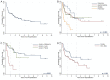
Results: Of 3137 patients treated with CPIs, 114 (3.6%) were hospitalized for irAEs, resulting in 124 hospitalizations. Gastrointestinal (GI)/hepatic, endocrine, and pulmonary irAEs were the most common causes of irAE-related hospitalization. After CPI initiation, the average time to hospitalization was 141 days. Median survival from hospital admission was 980 days. Patients hospitalized due to GI/hepatic and endocrine irAEs had longer median survival than patients with pulmonary irAEs (795 and 949 days vs. 83 days [P < .001]). Patients with melanoma and renal cell carcinoma had longer median survival than patients with lung cancer (2792 days and not reached vs. 159 days [P < .001]). There was longer median survival in the combination group compared to the PD-(L)1 group (1471 vs. 529 days [P = .04]).
Conclusions: As CPI use increases, irAE-related hospitalizations will as well. These findings suggest that among patients hospitalized for irAEs, survival differs by irAE and cancer type, with worse survival for patients with irAE pneumonitis or lung cancer. This real-world data contributes to research pertaining to hospitalization due to severe irAEs, which may inform patient counseling and treatment decision-making.
Keywords: cancer; hospitalization; immune checkpoint inhibitors; immunotherapy; irAE.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
Z.Q. recieved consulting fees from Novartis. S.B. recieved consulting fees from Balckstone and honorarium from System Analytic.
Figures
Similar articles
-
Improved survival and tumor control with Interleukin-2 is associated with the development of immune-related adverse events: data from the PROCLAIMSM registry.J Immunother Cancer. 2017 Dec 19;5(1):102. doi: 10.1186/s40425-017-0307-5. J Immunother Cancer. 2017. PMID: 29254506 Free PMC article. Clinical Trial.
-
Assessment of hospitalization rates for immune-related adverse events with immune checkpoint inhibitors.J Oncol Pharm Pract. 2021 Oct;27(7):1736-1742. doi: 10.1177/1078155220968909. Epub 2020 Oct 25. J Oncol Pharm Pract. 2021. PMID: 33100180
-
Prediction of severe immune-related adverse events requiring hospital admission in patients on immune checkpoint inhibitors: study of a population level insurance claims database from the USA.J Immunother Cancer. 2021 Mar;9(3):e001935. doi: 10.1136/jitc-2020-001935. J Immunother Cancer. 2021. PMID: 33789879 Free PMC article.
-
Analysis of the association between prospectively collected immune-related adverse events and survival in patients with solid tumor treated with immune-checkpoint blockers, taking into account immortal-time bias.Cancer Treat Rev. 2022 Nov;110:102452. doi: 10.1016/j.ctrv.2022.102452. Epub 2022 Aug 10. Cancer Treat Rev. 2022. PMID: 35998515 Review.
-
Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - A systematic review and meta-analysis.Cancer Treat Rev. 2021 Jan;92:102134. doi: 10.1016/j.ctrv.2020.102134. Epub 2020 Dec 3. Cancer Treat Rev. 2021. PMID: 33302134
Cited by
-
Impact of immune-related adverse events on survival among patients with head-and-neck squamous cell carcinoma.Immunotherapy. 2024;16(16-17):1069-1078. doi: 10.1080/1750743X.2024.2409617. Epub 2024 Oct 11. Immunotherapy. 2024. PMID: 39392156
-
Stocking the toolbox-Using preclinical models to understand the development and treatment of immune checkpoint inhibitor-induced immune-related adverse events.Immunol Rev. 2023 Sep;318(1):110-137. doi: 10.1111/imr.13250. Epub 2023 Aug 10. Immunol Rev. 2023. PMID: 37565407 Free PMC article. Review.
-
Immune-related adverse events requiring hospitalization in patients with lung cancer: implications and insights.Oncologist. 2024 Nov 4;29(11):e1615-e1620. doi: 10.1093/oncolo/oyae189. Oncologist. 2024. PMID: 39066589 Free PMC article.
-
Immunotherapy-Related Adverse Events and Clinical Outcomes in Adult Solid-Tumor Patients Admitted to an Onco-Hospitalist Medicine Service.Cancers (Basel). 2025 Jan 25;17(3):403. doi: 10.3390/cancers17030403. Cancers (Basel). 2025. PMID: 39941771 Free PMC article.
-
Latent profile analysis of self-management and its association with quality of life differences in patients with cancer treated with immune checkpoint inhibitors.Asia Pac J Oncol Nurs. 2025 Mar 13;12:100687. doi: 10.1016/j.apjon.2025.100687. eCollection 2025 Dec. Asia Pac J Oncol Nurs. 2025. PMID: 40271525 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

