Molecules that Generate Fingerprints: A New Class of Fluorescent Sensors for Chemical Biology, Medical Diagnosis, and Cryptography
- PMID: 37335975
- PMCID: PMC10324303
- DOI: 10.1021/acs.accounts.3c00162
Molecules that Generate Fingerprints: A New Class of Fluorescent Sensors for Chemical Biology, Medical Diagnosis, and Cryptography
Abstract
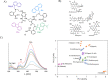
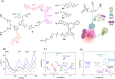
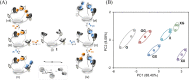
Fluorescent molecular sensors, often referred to as "turn-on" or "turn-off" fluorescent probes, are synthetic agents that change their fluorescence signal in response to analyte binding. Although these sensors have become powerful analytical tools in a wide range of research fields, they are generally limited to detecting only one or a few analytes. Pattern-generating fluorescent probes, which can generate unique identification (ID) fingerprints for different analytes, have recently emerged as a new class of luminescent sensors that can address this limitation. A unique characteristic of these probes, termed ID-probes, is that they integrate the qualities of conventional small-molecule-based fluorescent sensors and cross-reactive sensor arrays (often referred to as chemical, optical, or electronic noses/tongues). On the one hand, ID-probes can discriminate between various analytes and their combinations, akin to array-based analytical devices. On the other hand, their minute size enables them to analyze small-volume samples, track dynamic changes in a single solution, and operate in the microscopic world, which the macroscopic arrays cannot access.Here, we describe the principles underlying the ID-probe technology, as well as provide an overview of different ID-probes that have been developed to date and the ways they can be applied to a wide range of research fields. We describe, for example, ID-probes that can identify combinations of protein biomarkers in biofluids and in living cells, screen for several protein inhibitors simultaneously, analyze the content of Aβ aggregates, as well as ensure the quality of small-molecule and biological drugs. These examples highlight the relevance of this technology to medical diagnosis, bioassay development, cell and chemical biology, and pharmaceutical quality assurance, among others. ID-probes that can authorize users and protect secret data are also presented and the mechanisms that enable them to hide (steganography), encrypt (cryptography), and prevent access to (password protection) information are discussed.The versatility of this technology is further demonstrated by describing two types of probes: unimolecular ID-probes and self-assembled ID-probes. Probes from the first type can operate inside living cells, be recycled, and their initial patterns can be more easily obtained in a reproducible manner. The second type of probes can be readily modified and optimized, allowing one to prepare various different probes from a much wider range of fluorescent reporters and supramolecular recognition elements. Taken together, these developments indicate that the ID-probe sensing methodology is generally applicable, and that such probes can better characterize analyte mixtures or process chemically encoded information than can the conventional fluorescent molecular sensors. We therefore hope that this review will inspire the development of new types of pattern-generating probes, which would extend the fluorescence molecular toolbox currently used in the analytical sciences.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

