Modulating antibody effector functions by Fc glycoengineering
- PMID: 37336296
- PMCID: PMC11027751
- DOI: 10.1016/j.biotechadv.2023.108201
Modulating antibody effector functions by Fc glycoengineering
Abstract
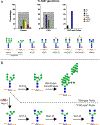
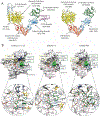
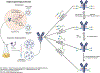
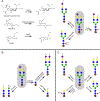
Antibody based drugs, including IgG monoclonal antibodies, are an expanding class of therapeutics widely employed to treat cancer, autoimmune and infectious diseases. IgG antibodies have a conserved N-glycosylation site at Asn297 that bears complex type N-glycans which, along with other less conserved N- and O-glycosylation sites, fine-tune effector functions, complement activation, and half-life of antibodies. Fucosylation, galactosylation, sialylation, bisection and mannosylation all generate glycoforms that interact in a specific manner with different cellular antibody receptors and are linked to a distinct functional profile. Antibodies, including those employed in clinical settings, are generated with a mixture of glycoforms attached to them, which has an impact on their efficacy, stability and effector functions. It is therefore of great interest to produce antibodies containing only tailored glycoforms with specific effects associated with them. To this end, several antibody engineering strategies have been developed, including the usage of engineered mammalian cell lines, in vitro and in vivo glycoengineering.
Keywords: Antibody; Endoglycosidase; Glycoengineering; Glycosynthase; IgG; N-glycosylation.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
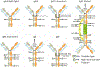
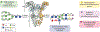

References
-
- Allen JG, Mujacic M, Frohn MJ, Pickrell AJ, Kodama P, Bagal D, San Miguel T, Sickmier EA, Osgood S, Swietlow A, Li V, Jordan JB, Kim K-W, Rousseau A-MC, Kim Y-J, Caille S, Achmatowicz M, Thiel O, Fotsch CH, Reddy P, McCarter JD, 2016. Facile modulation of antibody fucosylation with small molecule fucostatin inhibitors and cocrystal structure with GDP-mannose 4,6-dehydratase. ACS Chem Biol 11, 2734–2743. 10.1021/acschembio.6b00460 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

