c-Kit signaling potentiates CAR T cell efficacy in solid tumors by CD28- and IL-2-independent co-stimulation
- PMID: 37336986
- PMCID: PMC10765546
- DOI: 10.1038/s43018-023-00573-4
c-Kit signaling potentiates CAR T cell efficacy in solid tumors by CD28- and IL-2-independent co-stimulation
Abstract
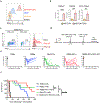
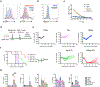
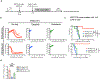
The limited efficacy of chimeric antigen receptor (CAR) T cell therapy for solid tumors necessitates engineering strategies that promote functional persistence in an immunosuppressive environment. Herein, we use c-Kit signaling, a physiological pathway associated with stemness in hematopoietic progenitor cells (T cells lose expression of c-Kit during differentiation). CAR T cells with intracellular expression, but no cell-surface receptor expression, of the c-Kit D816V mutation (KITv) have upregulated STAT phosphorylation, antigen activation-dependent proliferation and CD28- and interleukin-2-independent and interferon-γ-mediated co-stimulation, augmenting the cytotoxicity of first-generation CAR T cells. This translates to enhanced survival, including in transforming growth factor-β-rich and low-antigen-expressing solid tumor models. KITv CAR T cells have equivalent or better in vivo efficacy than second-generation CAR T cells and are susceptible to tyrosine kinase inhibitors (safety switch). When combined with CD28 co-stimulation, KITv co-stimulation functions as a third signal, enhancing efficacy and providing a potent approach to treat solid tumors.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Y.X. and P.S.A. have a pending patent application on the KITv mutation as a costimulatory domain to use in T cells. P.S.A. declares research funding from ATARA Biotherapeutics and Novocure; Scientific Advisory Board Member and Consultant for Adjuvant Genomics, ATARA Biotherapeutics, Abound Bio, Bio4t2, Carisma Therapeutics, Imugene, ImmPactBio, Johnston & Johnston, Link Immunotherapeutics, Orion pharma, Outpace Bio, Pluri-biotech, Putnam associates, Verismo Therapeutics; Patents, royalties, and intellectual property on MSLN-targeted CAR and other T-cell therapies licensed to ATARA Biotherapeutics, issued patent method for detection of cancer cells using virus, and pending patent applications on PD-1 dominant negative receptor, wireless pulse-oximetry device, and an ex vivo malignant pleural effusion culture system. All other authors do not have competing interests to disclose. Memorial Sloan Kettering Cancer Center has licensed intellectual property related to MSLN-targeted CARs and T-cell therapies to ATARA Biotherapeutics and has associated financial interests.
Figures



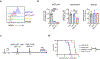

References
-
- Cappell KM & Kochenderfer JN A comparison of chimeric antigen receptors containing CD28 versus 4–1BB costimulatory domains. Nat Rev Clin Oncol 18, 715–727 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

