The role of cellular and molecular neuroimmune crosstalk in gut immunity
- PMID: 37336989
- PMCID: PMC10616093
- DOI: 10.1038/s41423-023-01054-5
The role of cellular and molecular neuroimmune crosstalk in gut immunity
Abstract
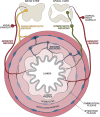
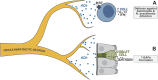
The gastrointestinal tract is densely innervated by the peripheral nervous system and populated by the immune system. These two systems critically coordinate the sensations of and adaptations to dietary, microbial, and damaging stimuli from the external and internal microenvironment during tissue homeostasis and inflammation. The brain receives and integrates ascending sensory signals from the gut and transduces descending signals back to the gut via autonomic neurons. Neurons regulate intestinal immune responses through the action of local axon reflexes or through neuronal circuits via the gut-brain axis. This neuroimmune crosstalk is critical for gut homeostatic maintenance and disease resolution. In this review, we discuss the roles of distinct types of gut-innervating neurons in the modulation of intestinal mucosal immunity. We will focus on the molecular mechanisms governing how different immune cells respond to neural signals in host defense and inflammation. We also discuss the therapeutic potential of strategies targeting neuroimmune crosstalk for intestinal diseases.
Keywords: Gut; Host defense; Neuroimmune; Neuroimmunology; Sensory.
© 2023. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
IMC consults for GSK pharmaceuticals and Limm Therapeutics and receives sponsored research support from Moderna, Inc.
Figures



Similar articles
-
Neuroimmune Crossroads: The Interplay of the Enteric Nervous System and Intestinal Macrophages in Gut Homeostasis and Disease.Biomolecules. 2024 Sep 2;14(9):1103. doi: 10.3390/biom14091103. Biomolecules. 2024. PMID: 39334870 Free PMC article. Review.
-
The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes.Mucosal Immunol. 2021 May;14(3):555-565. doi: 10.1038/s41385-020-00368-1. Epub 2021 Feb 4. Mucosal Immunol. 2021. PMID: 33542493 Free PMC article. Review.
-
The innervated gut and critical illness.Curr Opin Crit Care. 2025 Apr 1;31(2):198-203. doi: 10.1097/MCC.0000000000001260. Epub 2025 Feb 27. Curr Opin Crit Care. 2025. PMID: 40047233 Review.
-
Neuroimmune Interactions in the Intestine.Annu Rev Immunol. 2024 Jun;42(1):489-519. doi: 10.1146/annurev-immunol-101921-042929. Annu Rev Immunol. 2024. PMID: 38941607 Review.
-
Vagus nerve-mediated intestinal immune regulation: therapeutic implications of inflammatory bowel diseases.Int Immunol. 2022 Jan 22;34(2):97-106. doi: 10.1093/intimm/dxab039. Int Immunol. 2022. PMID: 34240133 Review.
Cited by
-
The Brain-Gut-Bone Axis in Neurodegenerative Diseases: Insights, Challenges, and Future Prospects.Adv Sci (Weinh). 2024 Oct;11(38):e2307971. doi: 10.1002/advs.202307971. Epub 2024 Aug 9. Adv Sci (Weinh). 2024. PMID: 39120490 Free PMC article. Review.
-
Microbiome: A Key Regulator of Body-Brain Interactions.Adv Exp Med Biol. 2025;1477:139-203. doi: 10.1007/978-3-031-89525-8_6. Adv Exp Med Biol. 2025. PMID: 40442386 Review.
-
Antimicrobial Neuropeptides and Their Receptors: Immunoregulator and Therapeutic Targets for Immune Disorders.Molecules. 2025 Jan 27;30(3):568. doi: 10.3390/molecules30030568. Molecules. 2025. PMID: 39942672 Free PMC article. Review.
-
Targeting the Gut-Brain Axis with Plant-Derived Essential Oils: Phytocannabinoids and Beyond.Nutrients. 2025 May 3;17(9):1578. doi: 10.3390/nu17091578. Nutrients. 2025. PMID: 40362887 Free PMC article. Review.
-
The progress of the microbe-gut-brain axis in sepsis-associated encephalopathy.Front Cell Infect Microbiol. 2025 May 13;15:1587463. doi: 10.3389/fcimb.2025.1587463. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40433666 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

