Paraoxonase 2 (PON2) plays a limited role in murine lung tumorigenesis
- PMID: 37337025
- PMCID: PMC10279720
- DOI: 10.1038/s41598-023-37146-5
Paraoxonase 2 (PON2) plays a limited role in murine lung tumorigenesis
Abstract
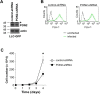
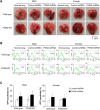
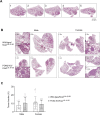
Paraoxonase 2 (PON2) is a multifunctional intracellular enzyme that has received growing attention for its ability to modulate various aspects of normal and malignant cellular physiology. Recent research has revealed that PON2 is upregulated in tissues from patients with various types of solid tumors and hematologic cancers, likely due to its ability to suppress oxidative stress and evade apoptosis. However, the effects of PON2 on pulmonary oncogenesis are unknown. Here, we conducted studies to investigate how PON2 influences lung cancer cell proliferation in vitro and lung tumorigenesis in vivo using a variety of cellular and animal models. It was found that PON2 expression deficiency hampered the proliferation of cultured lung cancer cells with concomitant cell cycle arrest at the G1 phase. In addition, the loss of endogenous PON2 expression impaired key aspects of oxidative metabolism in lung adenocarcinoma cells. Moreover, we investigated how the interplay between PON2 expression in lung tumors and host mice influences lung tumor initiation and progression. PON2 status in both transplanted tumor cells and mice failed to influence the development of subcutaneously grafted Lewis lung carcinoma (LLC) tumors, orthotopically implanted LLC tumors, and oncogenic Kras-driven primary lung adenocarcinoma tumors. Importantly, the frequencies of tumor-infiltrating myeloid subsets that include myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages were not impacted by PON2 expression in LLC tumor-bearing mice. Overall, our studies indicate that PON2 plays a limited role in murine lung tumorigenesis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
PON2 subverts metabolic gatekeeper functions in B cells to promote leukemogenesis.Proc Natl Acad Sci U S A. 2021 Feb 16;118(7):e2016553118. doi: 10.1073/pnas.2016553118. Proc Natl Acad Sci U S A. 2021. PMID: 33531346 Free PMC article.
-
Examining the role of paraoxonase 2 in the dopaminergic system of the mouse brain.BMC Neurosci. 2022 Sep 2;23(1):52. doi: 10.1186/s12868-022-00738-4. BMC Neurosci. 2022. PMID: 36056313 Free PMC article.
-
Exploring the role of paraoxonase-2 in bladder cancer: analyses performed on tissue samples, urines and cell cultures.Oncotarget. 2017 Apr 25;8(17):28785-28795. doi: 10.18632/oncotarget.15674. Oncotarget. 2017. PMID: 28430636 Free PMC article.
-
Paraoxonase-2 (PON2) in brain and its potential role in neuroprotection.Neurotoxicology. 2014 Jul;43:3-9. doi: 10.1016/j.neuro.2013.08.011. Epub 2013 Sep 4. Neurotoxicology. 2014. PMID: 24012887 Free PMC article. Review.
-
Insights into the role of paraoxonase 2 in human pathophysiology.J Biosci. 2021;46:4. J Biosci. 2021. PMID: 34987135 Review.
Cited by
-
Contribution of the Paraoxonase-2 Enzyme to Cancer Cell Metabolism and Phenotypes.Biomolecules. 2024 Feb 9;14(2):208. doi: 10.3390/biom14020208. Biomolecules. 2024. PMID: 38397445 Free PMC article. Review.
-
Proteogenomic characterization reveals tumorigenesis and progression of lung cancer manifested as subsolid nodules.Nat Commun. 2025 Mar 11;16(1):2414. doi: 10.1038/s41467-025-57364-x. Nat Commun. 2025. PMID: 40069142 Free PMC article.
-
Role of paraoxonase 2 (PON2) as a potential biomarker and therapeutic target in cancer treatment.J Cancer Res Clin Oncol. 2025 Aug 12;151(8):229. doi: 10.1007/s00432-025-06282-y. J Cancer Res Clin Oncol. 2025. PMID: 40794328 Free PMC article. Review.
-
Antiapoptotic PON2 expression and its clinical implications in locally advanced oral squamous cell carcinoma.Cancer Sci. 2024 Jun;115(6):2012-2022. doi: 10.1111/cas.16170. Epub 2024 Apr 11. Cancer Sci. 2024. PMID: 38602182 Free PMC article.
-
New Treatment Horizons in Uveal and Cutaneous Melanoma.Life (Basel). 2023 Jul 31;13(8):1666. doi: 10.3390/life13081666. Life (Basel). 2023. PMID: 37629523 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

