Immune response to COVID-19 vaccination in a population with a history of elevated exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water
- PMID: 37337047
- PMCID: PMC10541329
- DOI: 10.1038/s41370-023-00564-8
Immune response to COVID-19 vaccination in a population with a history of elevated exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water
Abstract
Background: Exposure to per- and polyfluoroalkyl substances (PFAS) has been linked to lower vaccine-induced antibody concentrations in children, while data from adults remains limited and equivocal. Characteristics of PFAS exposure and age at vaccination may modify such effects.
Objective: We used the mass administration of novel COVID-19 vaccines to test the hypothesis that prior exposure to environmentally-relevant concentrations of PFAS affect antibody response to vaccines in adolescents and adults.
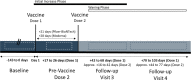
Methods: Between April and June 2021, 226 participants aged 12-90 years with a history of exposure to PFAS in drinking water and who received an mRNA COVID-19 vaccine participated in our prospective cohort study. SARS-CoV-2 anti-spike and anti-nucleocapsid antibodies (IgG) were quantified before the first and second vaccine doses and again at two follow-ups in the following months (up to 103 days post dose 1). Serum PFAS concentrations (n = 39 individual PFAS) were measured once for each participant during baseline, before their first vaccination. The association between PFAS exposure and immune response to vaccination was investigated using linear regression and generalized estimating equation (GEE) models with adjustment for covariates that affect antibody response. PFAS mixture effects were assessed using weighted quantile sum and Bayesian kernel machine regression methods.
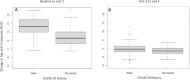
Results: The geometric mean (standard deviation) of perfluorooctane sulfonate and perfluorooctanoic acid serum concentrations in this population was 10.49 (3.22) and 3.90 (4.90) µg/L, respectively. PFAS concentrations were not associated with peak anti-spike antibody response, the initial increase in anti-spike antibody response following vaccination, or the waning over time of the anti-spike antibody response. Neither individual PFAS concentrations nor their evaluation as a mixture was associated with antibody response to mRNA vaccination against COVID-19.
Impact statement: Given the importance of understanding vaccine response among populations exposed to environmental contaminants and the current gaps in understanding this relationship outside of early life/childhood vaccinations, our manuscript contributes meaningful data from an adolescent and adult population receiving a novel vaccination.
Keywords: Emerging contaminants; Epidemiology; Health studies; PFAS.
© 2023. The Author(s).
Conflict of interest statement
DAS serves as a paid consultant to the Michigan Department of Health and Human Services and has served as a paid consultant in several legal cases involving PFAS and health. All other authors declare they have nothing to disclose.
Figures

References
-
- (ATSDR) AfTSaDR. PFAS in the US population | ATSDR. Agency for Toxic Substances and Disease Registry. 2022. https://www.atsdr.cdc.gov/pfas/health-effects/us-population.html
-
- Calafat AM, Kato K, Hubbard K, Jia T, Botelho JC, Wong LY. Legacy and alternative per- and polyfluoroalkyl substances in the U.S. general population: paired serum-urine data from the 2013-2014 National Health and Nutrition Examination Survey. Environ Int. 2019;131:105048. doi: 10.1016/j.envint.2019.105048. - DOI - PMC - PubMed
-
- Dassuncao C, Hu XC, Nielsen F, Weihe P, Grandjean P, Sunderland EM. Shifting global exposures to poly- and perfluoroalkyl substances (PFASs) evident in longitudinal birth cohorts from a seafood-consuming population. Environ Sci Technol. 2018;52:3738–47. doi: 10.1021/acs.est.7b06044. - DOI - PMC - PubMed
-
- Miaz LT, Plassmann MM, Gyllenhammar I, Bignert A, Sandblom O, Lignell S, et al. Temporal trends of suspect- and target-per/polyfluoroalkyl substances (PFAS), extractable organic fluorine (EOF) and total fluorine (TF) in pooled serum from first-time mothers in Uppsala, Sweden, 1996-2017. Environ Sci Process Impacts. 2020;22:1071–83. doi: 10.1039/c9em00502a. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

