Comparison of different second line treatments for metastatic pancreatic cancer: a systematic review and network meta-analysis
- PMID: 37337148
- PMCID: PMC10278314
- DOI: 10.1186/s12876-023-02853-w
Comparison of different second line treatments for metastatic pancreatic cancer: a systematic review and network meta-analysis
Abstract
Background: In metastatic pancreatic ductal adenocarcinoma (mPDAC), first line treatment options usually include combination regimens of folinic acid, 5-fluorouracil (5-FU), irinotecan, and oxaliplatin (FOLFIRINOX or mFOLFIRINOX) or gemcitabine based regimens such as in combination with albumin-bound paclitaxel (GEM + nab-PTX). After progression, multiple regimens including NALIRI + 5-FU and folinic acid, FOLFIRINOX, 5-FU-based oxaliplatin doublets (OFF, FOLFOX, or XELOX), or 5-FU-based monotherapy (FL, capecitabine, or S-1) are considered appropriate by major guidelines. This network meta-analysis (NMA) aimed to compare the efficacy of different treatment strategies tested as second-line regimens for patients with mPDAC after first-line gemcitabine-based systemic treatment.
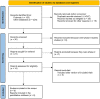
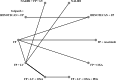
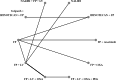
Methods: Randomized phase II and III clinical trials (RCTs) were included if they were published or presented in English. Trials of interest compared two active systemic treatments as second-line regimens until disease progression or unacceptable toxicity. We performed a Bayesian NMA with published hazard ratios (HRs) and 95%confidence intervals (CIs) to evaluate the comparative effectiveness of different second-line therapies for mPDAC. The main outcomes of interest were overall survival (OS) and progression free survival (PFS), secondary endpoints were grade 3-4 toxicities. We calculated the relative ranking of agents for each outcome as their surface under the cumulative ranking (SUCRA). A higher SUCRA score meant a higher ranking for efficacy outcomes.
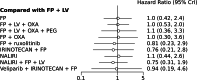
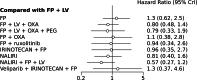
Results: A NMA of 9 treatments was performed for OS (n = 2521 patients enrolled). Compared with 5-FU + folinic acid both irinotecan or NALIRI + fluoropyrimidines had a trend to better OS (HR = 0.76, 95%CI 0.21-2.75 and HR = 0.74, 95%CI 0.31-1.85). Fluoropyrimidines + folinic acid + oxaliplatin were no better than the combination without oxaliplatin. The analysis of treatment ranking showed that the combination of NALIRI + 5-FU + folinic acid was most likely to yield the highest OS results (SUCRA = 0.7). Furthermore, the NMA results indicated that with the highest SUCRA score (SUCRA = 0.91), NALIRI + 5-FU + folinic acid may be the optimal choice for improved PFS amongst all regimens studied.
Conclusions: According to the NMA results, NALIRI + 5-FU, and folinic acid may represent the best second-line treatment for improved survival outcomes in mPDAC. Further evidence from prospective trials is needed to determine the best treatment option for this group of patients.
Keywords: 5-fluorouracil; Chemotherapy; Gemcitabine; NALIRI; Pancreatic adenocarcinoma; Second-line therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
The authors declare that they have no competing interests.
Figures
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Stat 2021 CACancer JClin. 2021;71:7–33. - PubMed
-
- Petrelli F et al. FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies.Pancreas. 2015 May;44(4):515–21. doi: 10.1097/MPA.0000000000000314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

