Pain assessment tools for use in infants: a meta-review
- PMID: 37337167
- PMCID: PMC10278280
- DOI: 10.1186/s12887-023-04099-7
Pain assessment tools for use in infants: a meta-review
Abstract
Background: Identifying pain in infants is challenging due to their inability to self-report pain, therefore the availability of valid and reliable means of assessing pain is critical.
Objective: This meta-review sought to identify evidence that could guide the selection of appropriate tools in this vulnerable population.
Methods: We searched Scopus, Medline, Embase, CINAHL, MIDRIS, EMCare and Google Scholar for eligible systematic reviews. Eligible reviews documented psychometric properties of available observational tools used to assess pain in infants.
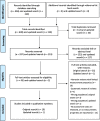
Results: A total of 516 reviews were identified of which 11 met our inclusion criteria. We identified 36 pain assessment tools (evaluated in 11 reviews) of which seven were reported in at least three reviews. The level of evidence reported on the psychometric properties of pain assessment tools varied widely ranging from low to good reliability and validity, whilst there are limited data on usability and clinical utility.
Conclusions: Currently, no observer administered pain assessment tool can be recommended as the gold standard due to limited availability and quality of the evidence that supports their validity, reliability and clinical utility. This meta-review attempts to collate the available evidence to assist clinicians to decide on what is the most appropriate tool to use in their clinical practice setting. It is important that researchers adopt a standard approach to evaluating the psychometric properties of pain assessment tools and evaluations of the clinical utility in order that the highest level of evidence can be used to guide tool selection.
Keywords: Clinical utility; Infants; Observational pain assessment tools; Reliability; Validity.
© 2023. The Author(s).
Conflict of interest statement
JDH was employed by PainChek Ltd as its Chief Scientific Officer in February 2020, previous to that JDH worked under a research contract between Curtin University and PainChek Ltd on the development and evaluation of PainChek’s Adult and Infant apps (formerly known as EPAT Technologies).
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous