Comparison of tramadol and lornoxicam for the prevention of postoperative catheter-related bladder discomfort: a randomized controlled trial
- PMID: 37337277
- PMCID: PMC10278340
- DOI: 10.1186/s13741-023-00317-z
Comparison of tramadol and lornoxicam for the prevention of postoperative catheter-related bladder discomfort: a randomized controlled trial
Abstract
Background: Catheter-related bladder discomfort (CRBD is a painful complication of intraoperative urinary catheterization after anaesthesia. We conducted this study to compare the effect of tramadol and lornoxicam for the prevention of postoperative CRBD.
Methods: One-hundred twenty patients (aged 18-60 years, ASA physical status 1-2, undergoing elective uterine surgery requiring intraoperative urinary catheterization were randomly divided into three groups with 40 patients in each group. Group T received 1.5 mg/kg tramadol, group L received 8-mg lornoxicam, and group C received normal saline. The study drugs were administered intravenously at the end of the surgery. The incidence and severity of CRBD were reported at 0, 1, 2, and 6 h after arrival at the postanaesthesia care unit (PACU).
Results: The incidence of CRBD was significantly lower in groups T and L than in group C at 1, 2, and 6 h after surgery. The incidence of moderate to severe CRBD was also significantly lower in groups T and L than in group C at 0, 1, and 2 h after surgery. The severity of CRBD reported as mild, moderate, and severe was reduced in groups T and L compared with group C at most times after surgery. Group T had a higher incidence of nausea than group C, and there were no differences in dizziness, drowsiness, or vomit among the three groups.
Conclusions: Tramadol and lornoxicam administered intravenously at the end of the surgery were both effective in preventing the incidence and severity of CRBD after uterine surgery. However, tramadol increased the incidence of nausea compared with saline, but there was no difference between tramadol and lornoxicam.
Trial registration: ChiCTR2100052003. Registered on 12/10/2021.
Keywords: Catheter-related bladder discomfort; Lornoxicam; Tramadol.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

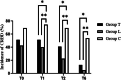


Similar articles
-
Comparison of intravenous lidocaine and dexmedetomidine infusion for prevention of postoperative catheter-related bladder discomfort: a randomized controlled trial.BMC Anesthesiol. 2019 Mar 18;19(1):37. doi: 10.1186/s12871-019-0708-8. BMC Anesthesiol. 2019. PMID: 30885134 Free PMC article. Clinical Trial.
-
Dorsal Penile Nerve Block With Ropivacaine-Reduced Postoperative Catheter-Related Bladder Discomfort in Male Patients After Emergence of General Anesthesia: A Prospective, Randomized, Controlled Study.Medicine (Baltimore). 2016 Apr;95(15):e3409. doi: 10.1097/MD.0000000000003409. Medicine (Baltimore). 2016. PMID: 27082620 Free PMC article. Clinical Trial.
-
Evaluation of intra-operative tramadol for prevention of catheter-related bladder discomfort: a prospective, randomized, double-blind study.Br J Anaesth. 2008 Oct;101(4):506-10. doi: 10.1093/bja/aen217. Epub 2008 Jul 24. Br J Anaesth. 2008. PMID: 18653496 Clinical Trial.
-
The systematic review and meta-analysis evaluated the efficacy and safety of nefopam for catheter-related bladder discomfort based on randomized controlled trials.Front Pharmacol. 2023 Nov 24;14:1305844. doi: 10.3389/fphar.2023.1305844. eCollection 2023. Front Pharmacol. 2023. PMID: 38074120 Free PMC article.
-
Comparative effectiveness of interventions for managing postoperative catheter-related bladder discomfort: a systematic review and network meta-analysis.J Anesth. 2019 Apr;33(2):197-208. doi: 10.1007/s00540-018-2597-2. Epub 2019 Jan 2. J Anesth. 2019. PMID: 30603826
Cited by
-
Dose-Response Analysis of Nalbuphine for Alleviating Catheter-Related Bladder Discomfort After Ureteroscopic Lithotripsy in Men: A Retrospective Study.Drug Des Devel Ther. 2025 Jun 19;19:5283-5292. doi: 10.2147/DDDT.S511613. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40552093 Free PMC article.
-
Use of disposable painless silicone urethral catheter during urological surgery for male patients: a randomized controlled study.World J Urol. 2025 Mar 31;43(1):199. doi: 10.1007/s00345-025-05506-7. World J Urol. 2025. PMID: 40164835 Clinical Trial.
References
-
- Andersson KE. The pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev. 1993;45(3):253–308. - PubMed
LinkOut - more resources
Full Text Sources

