Clinical outcomes in patients with metastatic castrate-resistant prostate cancer treated with abiraterone with or without ongoing androgen deprivation therapy: A retrospective case-control study
- PMID: 37337669
- PMCID: PMC10914526
- DOI: 10.1002/pros.24589
Clinical outcomes in patients with metastatic castrate-resistant prostate cancer treated with abiraterone with or without ongoing androgen deprivation therapy: A retrospective case-control study
Abstract
Introduction: Abiraterone and concurrent androgen deprivation therapy (ADT) are used in the treatment of patients with metastatic castration-resistant prostate cancer. Recently, it has been suggested that the use of abiraterone alone (without ADT) may have comparable efficacy to abiraterone with ongoing ADT. Here, we sought to assess the impact of ADT cessation in patients beginning abiraterone for castration-resistant prostate cancer.
Methods: We identified 39 patients at our institution who received abiraterone alone (with discontinuation of ADT) between 2011 and 2022. We then procured a comparable group of 39 patients (matched by age, Gleason score, and prostate-specific antigen [PSA] level) who received abiraterone with ongoing ADT during the same period. We assessed and compared clinical outcomes in the two groups (abiraterone-alone vs. abiraterone-ADT) with respect to PSA response rates, PSA progression-free survival, and overall survival. Results were adjusted using Cox proportional-hazards multivariable models.
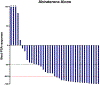
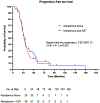
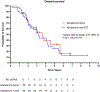
Results: The median PSA before treatment initiation was 12.7 (range: 0.2-199) ng/mL in the abiraterone-alone group and 15.5 (range: 0.6-212) ng/mL in the abiraterone-ADT group. Use of abiraterone alone adequately suppressed testosterone levels in 35/37 (94.6%) patients. Patients receiving abiraterone alone had a median PSA reduction of 80.2% versus 79.5% in patients receiving abiraterone plus ADT. The median PSA progression-free survival in patients receiving abiraterone alone was 27.4 versus 25.8 months in patients receiving abiraterone plus ADT (hazard ratio [HR] 1.10; 95% confidence interval [CI] 0.65-1.71; p = 0.82). In addition, abiraterone alone was associated with an overall survival of 3.6 versus 3.1 years in patients receiving abiraterone plus ADT (HR 0.90; 95% CI 0.50-1.62; p = 0.72). There were no differences in PFS or OS between groups after performing Cox multivariable regression analyses.
Conclusion: Use of abiraterone alone was associated with comparable clinical outcomes to patients who received abiraterone together with ADT. Further prospective studies are warranted to evaluate the impact of abiraterone alone on treatment outcomes and cost savings.
Keywords: ADT; abiraterone; overall survival; progression-free survival; retrospective case-control study.
© 2023 Wiley Periodicals LLC.
Figures

Similar articles
-
PSA provocation by bipolar androgen therapy may predict duration of response to first-line androgen deprivation: Updated results from the BATMAN study.Prostate. 2022 Dec;82(16):1529-1536. doi: 10.1002/pros.24426. Epub 2022 Aug 8. Prostate. 2022. PMID: 35938545 Free PMC article. Clinical Trial.
-
EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer.Eur Urol. 2014 Feb;65(2):467-79. doi: 10.1016/j.eururo.2013.11.002. Epub 2013 Nov 12. Eur Urol. 2014. PMID: 24321502
-
Adding abiraterone to androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: A systematic review and meta-analysis.Eur J Cancer. 2017 Oct;84:88-101. doi: 10.1016/j.ejca.2017.07.003. Epub 2017 Aug 8. Eur J Cancer. 2017. PMID: 28800492 Free PMC article.
-
Matching-adjusted indirect comparison of enzalutamide versus darolutamide doublet in mHSPC.Future Oncol. 2025 Aug;21(19):2459-2469. doi: 10.1080/14796694.2025.2526324. Epub 2025 Jul 14. Future Oncol. 2025. PMID: 40654300 Free PMC article. Clinical Trial.
-
Metformin for patients with metastatic prostate cancer starting androgen deprivation therapy: a randomised phase 3 trial of the STAMPEDE platform protocol.Lancet Oncol. 2025 Aug;26(8):1018-1030. doi: 10.1016/S1470-2045(25)00231-1. Epub 2025 Jul 7. Lancet Oncol. 2025. PMID: 40639383 Free PMC article. Clinical Trial.
Cited by
-
Case report: Exceptional and durable response to Radium-223 and suspension of androgen deprivation therapy in a metastatic castration-resistant prostate cancer patient.Front Oncol. 2024 Mar 8;14:1331643. doi: 10.3389/fonc.2024.1331643. eCollection 2024. Front Oncol. 2024. PMID: 38525428 Free PMC article.
References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023. Jan;73(1):17–48. - PubMed
-
- Schaeffer E, Srinivas S, Antonarakis ES, Armstrong AJ, Bekelman JE, Cheng H, et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J Natl Compr Canc Netw. 2021. Feb 2;19(2):134–43. - PubMed
-
- Scott LJ. Abiraterone Acetate: A Review in Metastatic Castration-Resistant Prostrate Cancer. Drugs. 2017. Sep;77(14):1565–76. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

