Commercial Immunoglobulin Products Contain Neutralizing Antibodies Against Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein
- PMID: 37338118
- PMCID: PMC10552578
- DOI: 10.1093/cid/ciad368
Commercial Immunoglobulin Products Contain Neutralizing Antibodies Against Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein
Abstract
Background: Patients with antibody deficiency respond poorly to coronavirus disease 2019 (COVID-19) vaccination and are at risk of severe or prolonged infection. They are given long-term immunoglobulin replacement therapy (IRT) prepared from healthy donor plasma to confer passive immunity against infection. Following widespread COVID-19 vaccination alongside natural exposure, we hypothesized that immunoglobulin preparations will now contain neutralizing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike antibodies, which confer protection against COVID-19 disease and may help to treat chronic infection.
Methods: We evaluated anti-SARS-CoV-2 spike antibody in a cohort of patients before and after immunoglobulin infusion. Neutralizing capacity of patient samples and immunoglobulin products was assessed using in vitro pseudovirus and live-virus neutralization assays, the latter investigating multiple batches against current circulating Omicron variants. We describe the clinical course of 9 patients started on IRT during treatment of COVID-19.
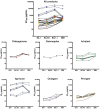
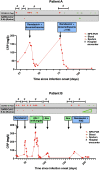
Results: In 35 individuals with antibody deficiency established on IRT, median anti-spike antibody titer increased from 2123 to 10 600 U/mL postinfusion, with corresponding increase in pseudovirus neutralization titers to levels comparable to healthy donors. Testing immunoglobulin products directly in the live-virus assay confirmed neutralization, including of BQ1.1 and XBB variants, but with variation between immunoglobulin products and batches.Initiation of IRT alongside remdesivir in patients with antibody deficiency and prolonged COVID-19 infection (median 189 days, maximum >900 days with an ancestral viral strain) resulted in clearance of SARS-CoV-2 at a median of 20 days.
Conclusions: Immunoglobulin preparations now contain neutralizing anti-SARS-CoV-2 antibodies that are transmitted to patients and help to treat COVID-19 in individuals with failure of humoral immunity.
Keywords: IVIG; SARS-CoV-2; immunodeficiency; neutralization; spike antibody.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. T. has received personal fees from CSL Behring and reports payment for attendance to an advisory board from CSL. S. W. has received personal fees from UCB and LFB Biopharmaceuticals; sponsorship to attend meetings from Biotest, CSL Behring, and Octapharma; grants or contracts from CSL Behring–International Nursing Group for Immunodeficiencies (INGID) for translation of INGID immunoglobulin administration guidelines and from BioCryst, Octapharma, CSL Behring, HIS, and Takeda for support for INGID conference 2022; honoraria from CSL Behring for clinicians’ experience using prefilled syringes; payment or honoraria from UCB for providing training for subcutaneous pump usage; support from Kenes–European Society of Immunodeficiency (ESID) conference organizers for attending ESID/INGID/International Patient Organisation for Primary Immunodeficiencies (IPOPI) conference; support from IPOPI for attending International Primary Immundoeficiencies Congress meeting; support from World Primary Immunodeficiencies Week (WPIW) for attending WPIW opening ceremony; and roles as board member of INGID, organizing committee for the International Primary Immunodeficiencies Congress, and steering committee for World PI Week. A. S. has received personal fees for a webinar from CSL Behring and sponsorship for conference from Takeda. S. O. B. has received grant support from CSL Behring to Royal Free London NHS Foundation Trust; grant funding to UCL from UCL Hospitals NHS Foundation Trust Biomedical Research Centers, Wellcome Trust, Medical Research Council, National Institute of Health Research, UK Research Institute, and Jeffrey Modell Foundation; consulting fees paid to UCL Consulting from GSK; personal honoraria for talks from Biotest; medicolegal fees related to primary immunodeficiency from various solicitor firms; personal fees or travel expenses from Immunodeficiency Canada/Israel Association of Allergy and Clinical Immunology, CSL Behring, Baxalta US, and Biotest; fees as an external expert for GSK; support for attending stakeholder meeting from IPOPI; participation on a data and safety monitoring board or advisory board for GSK (consultant fees paid to UCL Consulting) and NHS SCID Newborn Screening Diagnostic Reference Panel (unremunerated expert member); and unremunerated roles as Chair of the ESID clinical working party, board member of ESID, Chair for the European Reference Network for Rare Immunodeficiency, Autoinflammatory and Autoimmune diseases Transition Working Group, Chronic Granulomatous Disease Society research advisory panel, Associate Editor for Frontiers in Immunology, and Chronic Granulomatous Disease Society research advisory panel. D. M. L. has received personal fees from Gilead for an educational video on COVID-19 in immunodeficiency and from Merck for a roundtable discussion on risk of COVID-19 in immunosuppressed patients; speaker’s fees from Biotest; support to attend a conference from Octapharma; research grants from GSK and Bristol Myers Squibb, outside the current work, paid to institution; consulting fees from GSK, paid to institution; payment or honoraria for roundtable discussion on COVID-19 in immunosuppressed patients from Merck; speaker’s fees from Biotest; travel and accommodation fees for ESID conference 2022 from Octapharma; and a role as member of NHS England Expert Working group on COVID-19 therapeutics and independent advisory group on high-risk patients. R. C. L. B. reports ad hoc consultancy fees paid to author, unrelated to this manuscript, from Immunos Therapeutics, and unremunerated participation as member of an independent advisory group on COVID Therapeutics (and other similar advisory groups). E. J. C. reports unsalaried membership of the UK Kidney Association Infection Prevention and Control Committee. S. T. reports support for attending a conference including travel to meeting from CSL and support for attending a meeting from Takeda. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

Comment in
-
Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Replacement Therapy for Immunocompromised Patients.Clin Infect Dis. 2023 Oct 5;77(7):961-963. doi: 10.1093/cid/ciad367. Clin Infect Dis. 2023. PMID: 37337905 No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

