Enhanced Virus Translation Enables miR-122-Independent Hepatitis C Virus Propagation
- PMID: 37338370
- PMCID: PMC10373559
- DOI: 10.1128/jvi.00858-21
Enhanced Virus Translation Enables miR-122-Independent Hepatitis C Virus Propagation
Abstract
The 5' untranslated region (UTR) of the hepatitis C virus (HCV) genome forms RNA structures that regulate virus replication and translation. The region contains an internal ribosomal entry site (IRES) and a 5'-terminal region. Binding of the liver-specific microRNA (miRNA) miR-122 to two binding sites in the 5'-terminal region regulates viral replication, translation, and genome stability and is essential for efficient virus replication, but its precise mechanism of action is still unresolved. A current hypothesis is that miR-122 binding stimulates viral translation by facilitating the viral 5' UTR to form the translationally active HCV IRES RNA structure. While miR-122 is essential for detectable replication of wild-type HCV genomes in cell culture, several viral variants with 5' UTR mutations exhibit low-level replication in the absence of miR-122. We show that HCV mutants capable of replicating independently of miR-122 display an enhanced translation phenotype that correlates with their ability to replicate independently of miR-122. Further, we provide evidence that translation regulation is the major role for miR-122 and show that miR-122-independent HCV replication can be rescued to miR-122-dependent levels by the combined impacts of 5' UTR mutations that stimulate translation and by stabilizing the viral genome by knockdown of host exonucleases and phosphatases that degrade the genome. Finally, we show that HCV mutants capable of replicating independently of miR-122 also replicate independently of other microRNAs generated by the canonical miRNA synthesis pathway. Thus, we provide a model suggesting that translation stimulation and genome stabilization are the primary roles for miR-122 in promoting HCV. IMPORTANCE The unusual and essential role of miR-122 in promoting HCV propagation is incompletely understood. To better understand its role, we have analyzed HCV mutants capable of replicating independently of miR-122. Our data show that the ability of viruses to replicate independently of miR-122 correlates with enhanced virus translation but that genome stabilization is required to restore efficient HCV replication. This suggests that viruses must gain both abilities to escape the need for miR-122 and impacts the possibility that HCV can evolve to replicate outside the liver.
Keywords: 5′ UTR; 5′ untranslated region; HCV; genome stability; hepatitis C virus; miR-122; miR-122-independent replication; translation; viral translation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
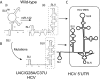
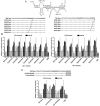


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

