A Phase 2 Randomized Trial Evaluating the Antiviral Activity and Safety of the Direct-Acting Antiviral Bemnifosbuvir in Ambulatory Patients with Mild or Moderate COVID-19 (MOONSONG Study)
- PMID: 37338393
- PMCID: PMC10434175
- DOI: 10.1128/spectrum.00077-23
A Phase 2 Randomized Trial Evaluating the Antiviral Activity and Safety of the Direct-Acting Antiviral Bemnifosbuvir in Ambulatory Patients with Mild or Moderate COVID-19 (MOONSONG Study)
Abstract
Bemnifosbuvir is an oral antiviral drug with a dual mechanism of action targeting viral RNA polymerase, with in vitro activity against SARS-CoV-2. We conducted a phase 2, double-blind study evaluating the antiviral activity, safety, efficacy, and pharmacokinetics of bemnifosbuvir in ambulatory patients with mild/moderate COVID-19. Patients were randomized 1:1 to bemnifosbuvir 550 mg or placebo (cohort A) and 3:1 to bemnifosbuvir 1,100 mg or placebo (cohort B); all doses were given twice daily for 5 days. The primary endpoint was a change from baseline in the amount of nasopharyngeal SARS-CoV-2 viral RNA by reverse transcription PCR (RT-PCR). The modified intent-to-treat infected population comprised 100 patients (bemnifosbuvir 550 mg, n = 30; bemnifosbuvir 1,100 mg, n = 30; cohort A placebo, n = 30; cohort B placebo, n = 10). The primary endpoint was not met: the difference in viral RNA adjusted means at day 7 was -0.25 log10 copies/mL between bemnifosbuvir 550 mg and cohort A placebo (80% confidence interval [CI], -0.66 to 0.16; P = 0.4260), and -0.08 log10 copies/mL between bemnifosbuvir 1,100 mg and pooled placebo (80% CI, -0.48 to 0.33; P = 0.8083). Bemnifosbuvir 550 mg was well tolerated. Incidence of nausea and vomiting was higher with bemnifosbuvir 1,100 mg (10.0% and 16.7% of patients, respectively) than pooled placebo (2.5% nausea, 2.5% vomiting). In the primary analysis, bemnifosbuvir did not show meaningful antiviral activity on nasopharyngeal viral load as measured by RT-PCR compared with placebo in patients with mild/moderate COVID-19. The trial is registered at ClinicalTrials.gov under registration number NCT04709835. IMPORTANCE COVID-19 continues to be a major global public health challenge, and there remains a need for effective and convenient direct-acting antivirals that can be administered outside health care settings. Bemnifosbuvir is an oral antiviral with a dual mechanism of action and potent in vitro activity against SARS-CoV-2. In this study, we evaluated the antiviral activity, safety, efficacy, and pharmacokinetics of bemnifosbuvir in ambulatory patients with mild/moderate COVID-19. In the primary analysis, bemnifosbuvir did not show meaningful antiviral activity compared with placebo as assessed by nasopharyngeal viral loads. The negative predictive value of nasopharyngeal viral load reduction for clinical outcomes in COVID-19 is currently unclear, and further evaluation of bemnifosbuvir for COVID-19 may be warranted despite the findings observed in this study.
Keywords: AT-527; COVID-19; SARS-CoV-2; bemnifosbuvir; direct-acting antiviral.
Conflict of interest statement
The authors declare a conflict of interest. This work was supported by F. Hoffmann-La Roche Ltd and Atea Pharmaceuticals. The study was designed and co-funded by F. Hoffmann-La Roche Ltd and Atea Pharmaceuticals. Third-party medical writing assistance was provided by Beth de Klerk, of Ashfield MedComms, an Inizio company, and was funded by F. Hoffmann-La Roche Ltd. M.B. has been an advisor for and received travel and research grants (to the organization) from Janssen, Roche, ViiV, Bristol-Myers Squibb, Pfizer, Merck Sharp & Dohme, Gilead, Mylan, Cipla, Teva, GSK, Novavax, and Valneva. E.D.: No conflict. K.S.: No conflict. W.H., B.C., N.C., and V.U. are employees of Roche Products Ltd and hold shares of F. Hoffmann-La Roche Ltd. S.W. is an employee and holds shares of F. Hoffmann-La Roche Ltd. A.H., K.P., and X.-J.Z. are employees of Atea Pharmaceuticals.
Figures
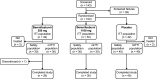
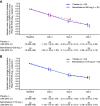
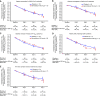

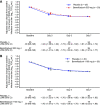

References
-
- Johns Hopkins University. 2022. COVID-19 dashboard. https://coronavirus.jhu.edu/map.html. Accessed 3 October 2022.
-
- Paredes MI, Lunn SM, Famulare M, Frisbie LA, Painter I, Burstein R, Roychoudhury P, Xie H, Mohamed Bakhash SA, Perez R, Lukes M, Ellis S, Sathees S, Mathias PC, Greninger A, Starita LM, Frazar CD, Ryke E, Zhong W, Gamboa L, Threlkeld M, Lee J, McDermot E, Truong M, Nickerson DA, Bates DL, Hartman ME, Haugen E, Nguyen TN, Richards JD, Rodriguez JL, Stamatoyannopoulos JA, Thorland E, Melly G, Dykema PE, MacKellar DC, Gray HK, Singh A, Peterson JM, Russell D, Torres LM, Lindquist S, Bedford T, Allen KJ, Oltean HN. 2022. Associations between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants and risk of coronavirus disease 2019 (COVID-19) hospitalization among confirmed cases in Washington state: a retrospective cohort study. Clin Infect Dis 75:e536–e544. doi:10.1093/cid/ciac279. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention. 2022. Symptoms of COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed 3 October 2022.
-
- European Medicines Agency. COVID-19 vaccines: key facts. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-thr.... Accessed 3 October 2021.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

