Polydom/SVEP1 binds to Tie1 and promotes migration of lymphatic endothelial cells
- PMID: 37338522
- PMCID: PMC10281526
- DOI: 10.1083/jcb.202208047
Polydom/SVEP1 binds to Tie1 and promotes migration of lymphatic endothelial cells
Abstract
Polydom is an extracellular matrix protein involved in lymphatic vessel development. Polydom-deficient mice die immediately after birth due to defects in lymphatic vessel remodeling, but the mechanism involved is poorly understood. Here, we report that Polydom directly binds to Tie1, an orphan receptor in the Angiopoietin-Tie axis, and facilitates migration of lymphatic endothelial cells (LECs) in a Tie1-dependent manner. Polydom-induced LEC migration is diminished by PI3K inhibitors but not by an ERK inhibitor, suggesting that the PI3K/Akt signaling pathway is involved in Polydom-induced LEC migration. In line with this possibility, Akt phosphorylation in LECs is enhanced by Polydom although no significant Tie1 phosphorylation is induced by Polydom. LECs also exhibited nuclear exclusion of Foxo1, a signaling event downstream of Akt activation, which was impaired in Polydom-deficient mice. These findings indicate that Polydom is a physiological ligand for Tie1 and participates in lymphatic vessel development through activation of the PI3K/Akt pathway.
© 2023 Sato-Nishiuchi et al.
Conflict of interest statement
Disclosures: K. Sekiguchi reported personal fees from Matrixome, Inc., “other” from Nippi, Inc., grants from Mandom, Inc., and grants from Kao Corporation outside the submitted work. No other disclosures were reported.
Figures


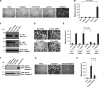
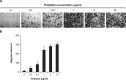


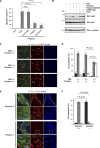
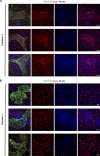

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

