The additive effect of vitamin K supplementation and bisphosphonate on fracture risk in post-menopausal osteoporosis: a randomised placebo controlled trial
- PMID: 37338608
- PMCID: PMC10282078
- DOI: 10.1007/s11657-023-01288-w
The additive effect of vitamin K supplementation and bisphosphonate on fracture risk in post-menopausal osteoporosis: a randomised placebo controlled trial
Abstract
This study assessed whether vitamin K, given with oral bisphosphonate, calcium and/or vitamin D has an additive effect on fracture risk in post-menopausal women with osteoporosis. No difference in bone density or bone turnover was observed although vitamin K1 supplementation led to a modest effect on parameters of hip geometry.
Purpose: Some clinical studies have suggested that vitamin K prevents bone loss and may improve fracture risk. The aim was to assess whether vitamin K supplementation has an additive effect on bone mineral density (BMD), hip geometry and bone turnover markers (BTMs) in post-menopausal women with osteoporosis (PMO) and sub-optimum vitamin K status receiving bisphosphonate, calcium and/or vitamin D treatment.
Methods: We conducted a trial in 105 women aged 68.7[12.3] years with PMO and serum vitamin K1 ≤ 0.4 µg/L. They were randomised to 3 treatment arms; vitamin K1 (1 mg/day) arm, vitamin K2 arm (MK-4; 45 mg/day) or placebo for 18 months. They were on oral bisphosphonate and calcium and/or vitamin D. We measured BMD by DXA, hip geometry parameters using hip structural analysis (HSA) software and BTMs. Vitamin K1 or MK-4 supplementation was each compared to placebo. Intention to treat (ITT) and per protocol (PP) analyses were performed.
Results: Changes in BMD at the total hip, femoral neck and lumbar spine and BTMs; CTX and P1NP did not differ significantly following either K1 or MK-4 supplementation compared to placebo. Following PP analysis and correction for covariates, there were significant differences in some of the HSA parameters at the intertrochanter (IT) and femoral shaft (FS): IT endocortical diameter (ED) (% change placebo:1.5 [4.1], K1 arm: -1.02 [5.07], p = 0.04), FS subperiosteal/outer diameter (OD) (placebo: 1.78 [5.3], K1 arm: 0.46 [2.23] p = 0.04), FS cross sectional area (CSA) (placebo:1.47 [4.09],K1 arm: -1.02[5.07], p = 0.03).
Conclusion: The addition of vitamin K1 to oral bisphosphonate with calcium and/or vitamin D treatment in PMO has a modest effect on parameters of hip geometry. Further confirmatory studies are needed.
Trial registration: The study was registered at Clinicaltrial.gov:NCT01232647.
Keywords: Bisphosphonate; Hip geometry; Post menopausal osteoporosis; Vitamin K.
© 2023. The Author(s).
Conflict of interest statement
"Amelia Moore, Dwight Dulnoan, Kieran Voong, Salma Ayis, Anastasios Mangelis, Renata Gorska, Dominic. J. Harrington and Jonathan CY Tang declare that they have no conflict of interests’.
William D. Fraser has received past educational grants, speaker honoraria and been on advisory boards in relation to Vitamin D and all treatments for osteoporosis.
Geeta Hampson has received speaker honoraria and been on advisory boards in relation to Vitamin D and all treatments for osteoporosis.
Figures
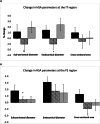
 , Vitamin K1 arm:
, Vitamin K1 arm:
 , MK-4 arm:
, MK-4 arm:
 , * p < 0.05 compared to placebo, #
p = 0.06 corrected for covariates in exploratory analyses. Results are expressed as mean [SEM]
, * p < 0.05 compared to placebo, #
p = 0.06 corrected for covariates in exploratory analyses. Results are expressed as mean [SEM]References
-
- NOGG (2021) Clinical guideline for the prevention and treatment of osteoporosis 2021. https://www.nogg.org.uk/sites/nogg/download/NOGG-Guideline-2021-g.pdf
-
- Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Welch V, Coyle D, Tugwell P (2008) Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev (1):CD001155. 10.1002/14651858.CD001155 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

