Safety and Efficacy of ApTOLL in Patients With Ischemic Stroke Undergoing Endovascular Treatment: A Phase 1/2 Randomized Clinical Trial
- PMID: 37338893
- PMCID: PMC10282959
- DOI: 10.1001/jamaneurol.2023.1660
Safety and Efficacy of ApTOLL in Patients With Ischemic Stroke Undergoing Endovascular Treatment: A Phase 1/2 Randomized Clinical Trial
Erratum in
-
Error in Author Surname.JAMA Neurol. 2024 Feb 26;81(4):425. doi: 10.1001/jamaneurol.2024.0165. Online ahead of print. JAMA Neurol. 2024. PMID: 38407880 Free PMC article. No abstract available.
Abstract
Importance: ApTOLL is a TLR4 antagonist with proven preclinical neuroprotective effect and a safe profile in healthy volunteers.
Objective: To assess the safety and efficacy of ApTOLL in combination with endovascular treatment (EVT) for patients with ischemic stroke.
Design, setting, and participants: This phase 1b/2a, double-blind, randomized, placebo-controlled study was conducted at 15 sites in Spain and France from 2020 to 2022. Participants included patients aged 18 to 90 years who had ischemic stroke due to large vessel occlusion and were seen within 6 hours after stroke onset; other criteria were an Alberta Stroke Program Early CT Score of 6 to 10, estimated infarct core volume on baseline computed tomography perfusion of 5 to 70 mL, and the intention to undergo EVT. During the study period, 4174 patients underwent EVT.
Interventions: In phase 1b, 0.025, 0.05, 0.1, or 0.2 mg/kg of ApTOLL or placebo; in phase 2a, 0.05 or 0.2 mg/kg of ApTOLL or placebo; and in both phases, treatment with EVT and intravenous thrombolysis if indicated.
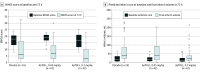
Main outcomes and measures: The primary end point was the safety of ApTOLL based on death, symptomatic intracranial hemorrhage (sICH), malignant stroke, and recurrent stroke. Secondary efficacy end points included final infarct volume (via MRI at 72 hours), NIHSS score at 72 hours, and disability at 90 days (modified Rankin Scale [mRS] score).
Results: In phase Ib, 32 patients were allocated evenly to the 4 dose groups. After phase 1b was completed with no safety concerns, 2 doses were selected for phase 2a; these 119 patients were randomized to receive ApTOLL, 0.05 mg/kg (n = 36); ApTOLL, 0.2 mg/kg (n = 36), or placebo (n = 47) in a 1:1:√2 ratio. The pooled population of 139 patients had a mean (SD) age of 70 (12) years, 81 patients (58%) were male, and 58 (42%) were female. The primary end point occurred in 16 of 55 patients (29%) receiving placebo (10 deaths [18.2%], 4 sICH [7.3%], 4 malignant strokes [7.3%], and 2 recurrent strokes [3.6%]); in 15 of 42 patients (36%) receiving ApTOLL, 0.05 mg/kg (11 deaths [26.2%], 3 sICH [7.2%], 2 malignant strokes [4.8%], and 2 recurrent strokes [4.8%]); and in 6 of 42 patients (14%) receiving ApTOLL, 0.2 mg/kg (2 deaths [4.8%], 2 sICH [4.8%], and 3 recurrent strokes [7.1%]). ApTOLL, 0.2 mg/kg, was associated with lower NIHSS score at 72 hours (mean difference log-transformed vs placebo, -45%; 95% CI, -67% to -10%), smaller final infarct volume (mean difference log-transformed vs placebo, -42%; 95% CI, -66% to 1%), and lower degrees of disability at 90 days (common odds ratio for a better outcome vs placebo, 2.44; 95% CI, 1.76 to 5.00).
Conclusions and relevance: In acute ischemic stroke, 0.2 mg/kg of ApTOLL administered within 6 hours of onset in combination with EVT was safe and associated with a potential meaningful clinical effect, reducing mortality and disability at 90 days compared with placebo. These preliminary findings await confirmation from larger pivotal trials.
Trial registration: ClinicalTrials.gov Identifier: NCT04734548.
Conflict of interest statement
Figures