Multiple Pharmacotherapy Adaptations for Smoking Cessation Based on Treatment Response in Black Adults Who Smoke: A Randomized Clinical Trial
- PMID: 37338906
- PMCID: PMC10282892
- DOI: 10.1001/jamanetworkopen.2023.17895
Multiple Pharmacotherapy Adaptations for Smoking Cessation Based on Treatment Response in Black Adults Who Smoke: A Randomized Clinical Trial
Abstract
Importance: Adapting to different smoking cessation medications when an individual has not stopped smoking has shown promise, but efficacy has not been tested in racial and ethnic minority individuals who smoke and tend to have less success in quitting and bear a disproportionate share of tobacco-related morbidity and mortality.
Objective: To evaluate efficacy of multiple smoking cessation pharmacotherapy adaptations based on treatment response in Black adults who smoke daily.
Design, setting, and participants: This randomized clinical trial of adapted therapy (ADT) or enhanced usual care (UC) included non-Hispanic Black adults who smoke and was conducted from May 2019 to January 2022 at a federally qualified health center in Kansas City, Missouri. Data analysis took place from March 2022 to January 2023.
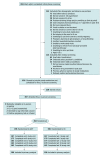
Interventions: Both groups received 18 weeks of pharmacotherapy with long-term follow-up through week 26. The ADT group consisted of 196 individuals who received a nicotine patch (NP) and up to 2 pharmacotherapy adaptations, with a first switch to varenicline at week 2 and, if needed, a second switch to bupropion plus NP (bupropion + NP) based on carbon monoxide (CO)-verified smoking status (CO ≥6 ppm) at week 6. The UC group consisted of 196 individuals who received NP throughout the duration of treatment.
Main outcomes and measures: Anabasine-verified and anatabine-verified point-prevalence abstinence at week 12 (primary end point) and weeks 18 and 26 (secondary end points). The χ2 test was used to compare verified abstinence at week 12 (primary end point) and weeks 18 and 26 (secondary end points) between ADT and UC. A post hoc sensitivity analysis of smoking abstinence at week 12 was performed with multiple imputation using a monotone logistic regression with treatment and gender as covariates to impute the missing data.
Results: Among 392 participants who were enrolled (mean [SD] age, 53 [11.6] years; 224 [57%] female; 186 [47%] ≤ 100% federal poverty level; mean [SD] 13 [12.4] cigarettes per day), 324 (83%) completed the trial. Overall, 196 individuals were randomized to each study group. Using intent-to-treat and imputing missing data as participants who smoke, verified 7-day abstinence was not significantly different by treatment group at 12 weeks (ADT: 34 of 196 [17.4%]; UC: 23 of 196 [11.7%]; odds ratio [OR], 1.58; 95% CI, 0.89-2.80; P = .12), 18 weeks (ADT: 32 of 196 [16.3%]; UC: 31 of 196 [15.8%]; OR, 1.04; 95% CI, 0.61-1.78; P = .89), and 26 weeks (ADT: 24 of 196 [12.2%]; UC: 26 of 196 [13.3%]; OR, 0.91; 95% CI, 0.50-1.65; P = .76). Of the ADT participants who received pharmacotherapy adaptations (135/188 [71.8%]), 11 of 135 (8.1%) were abstinent at week 12. Controlling for treatment, individuals who responded to treatment and had CO-verified abstinence at week 2 had 4.6 times greater odds of being abstinent at week 12 (37 of 129 [28.7%] abstinence) than those who did not respond to treatment (19 of 245 [7.8%] abstinence; OR; 4.6; 95% CI, 2.5-8.6; P < .001).
Conclusions and relevance: In this randomized clinical trial of adapted vs standard of care pharmacotherapy, adaptation to varenicline and/or bupropion + NP after failure of NP monotherapy did not significantly improve abstinence rates for Black adults who smoke relative to those who continued treatment with NP. Those who achieved abstinence in the first 2 weeks of the study were significantly more likely to achieve later abstinence, highlighting early treatment response as an important area for preemptive intervention.
Trial registration: ClinicalTrials.gov Identifier: NCT03897439.
Conflict of interest statement
Figures

Similar articles
-
A review of the efficacy of smoking-cessation pharmacotherapies in nonwhite populations.Clin Ther. 2008 May;30(5):800-12. doi: 10.1016/j.clinthera.2008.05.010. Clin Ther. 2008. PMID: 18555928 Review.
-
Assessment of Racial Differences in Pharmacotherapy Efficacy for Smoking Cessation: Secondary Analysis of the EAGLES Randomized Clinical Trial.JAMA Netw Open. 2021 Jan 4;4(1):e2032053. doi: 10.1001/jamanetworkopen.2020.32053. JAMA Netw Open. 2021. PMID: 33464316 Free PMC article. Clinical Trial.
-
Adaptive Smoking Cessation Using Precessation Varenicline or Nicotine Patch: A Randomized Clinical Trial.JAMA Netw Open. 2023 Sep 5;6(9):e2332214. doi: 10.1001/jamanetworkopen.2023.32214. JAMA Netw Open. 2023. PMID: 37682573 Free PMC article. Clinical Trial.
-
Financial Incentives for Smoking Cessation Among Socioeconomically Disadvantaged Adults: A Randomized Clinical Trial.JAMA Netw Open. 2024 Jul 1;7(7):e2418821. doi: 10.1001/jamanetworkopen.2024.18821. JAMA Netw Open. 2024. PMID: 38954415 Free PMC article. Clinical Trial.
-
Antidepressants for smoking cessation.Cochrane Database Syst Rev. 2020 Apr 22;4(4):CD000031. doi: 10.1002/14651858.CD000031.pub5. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 May 24;5:CD000031. doi: 10.1002/14651858.CD000031.pub6. PMID: 32319681 Free PMC article. Updated.
Cited by
-
Gaps in smoking cessation counseling administered by healthcare providers to BIPOC gay men who smoke daily in the U.S.J Subst Use Addict Treat. 2025 Feb;169:209590. doi: 10.1016/j.josat.2024.209590. Epub 2024 Nov 30. J Subst Use Addict Treat. 2025. PMID: 39622436
-
Early Treatment Response in Black Smokers Undergoing Pharmacotherapy for Smoking Cessation: A Secondary Analysis of a Randomized Clinical Trial.JAMA Netw Open. 2023 Sep 5;6(9):e2334695. doi: 10.1001/jamanetworkopen.2023.34695. JAMA Netw Open. 2023. PMID: 37728930 Free PMC article. Clinical Trial.
References
-
- US National Cancer Institute. A socioecological approach to addressing tobacco-related health disparities. National Cancer Institute Tobacco Control Monograph 22. Accessed May 4, 2023. https://cancercontrol.cancer.gov/sites/default/files/2020-08/m22_complet...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

