Metal-Complexes Bearing Releasable CO Differently Modulate Amyloid Aggregation
- PMID: 37338927
- PMCID: PMC10324395
- DOI: 10.1021/acs.inorgchem.3c01522
Metal-Complexes Bearing Releasable CO Differently Modulate Amyloid Aggregation
Abstract
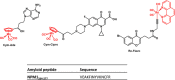
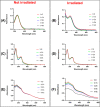
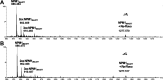
Neurodegenerative diseases are often associated with an uncontrolled amyloid aggregation. Hence, many studies are oriented to discover new compounds that are able to modulate self-recognition mechanisms of proteins involved in the development of these pathologies. Herein, three metal-complexes able to release carbon monoxide (CORMs) were analyzed for their ability to affect the self-aggregation of the amyloidogenic fragment of nucleophosmin 1, corresponding to the second helix of the three-helix bundle located in the C-terminal domain of the protein, i.e., NPM1264-277, peptide. These complexes were two cymantrenes coordinated to the nucleobase adenine (Cym-Ade) and to the antibiotic ciprofloxacin (Cym-Cipro) and a Re(I)-compound containing 1,10-phenanthroline and 3-CCCH2NHCOCH2CH2-6-bromo-chromone as ligands (Re-Flavo). Thioflavin T (ThT) assay, UV-vis absorption and fluorescence spectroscopies, scanning electron microscopy (SEM), and electrospray ionization mass spectrometry (ESI-MS) indicated that the three compounds have different effects on the peptide aggregation. Cym-Ade and Cym-Cipro act as aggregating agents. Cym-Ade induces the formation of NPM1264-277 fibers longer and stiffer than that formed by NPM1264-277 alone; irradiation of complexes speeds the formation of fibers that are more flexible and thicker than those found without irradiation. Cym-Cipro induces the formation of longer fibers, although slightly thinner in diameter. Conversely, Re-Flavo acts as an antiaggregating agent. Overall, these results indicate that metal-based CORMs with diverse structural features can have a different effect on the formation of amyloid fibers. A proper choice of ligands attached to metal can allow the development of metal-based drugs with potential application as antiamyloidogenic agents.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



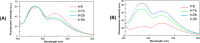


Similar articles
-
A Half-Sandwich Os(II) Glucoconjugated NHC Complex as a Modulator of Amyloid Aggregation.Inorg Chem. 2025 Feb 24;64(7):3335-3345. doi: 10.1021/acs.inorgchem.4c04823. Epub 2025 Feb 13. Inorg Chem. 2025. PMID: 39945504 Free PMC article.
-
Heterobimetallic ferrocenyl-chromone compounds as selective inhibitors of amyloid aggregation.J Inorg Biochem. 2025 Sep;270:112932. doi: 10.1016/j.jinorgbio.2025.112932. Epub 2025 Apr 23. J Inorg Biochem. 2025. PMID: 40288002
-
Glucosyl Platinum(II) Complexes Inhibit Aggregation of the C-Terminal Region of the Aβ Peptide.Inorg Chem. 2022 Feb 28;61(8):3540-3552. doi: 10.1021/acs.inorgchem.1c03540. Epub 2022 Feb 16. Inorg Chem. 2022. PMID: 35171608 Free PMC article.
-
Rational design of photoactivatable metal complexes to target and modulate amyloid-β peptides.J Inorg Biochem. 2023 Jan;238:112053. doi: 10.1016/j.jinorgbio.2022.112053. Epub 2022 Oct 28. J Inorg Biochem. 2023. PMID: 36347209 Review.
-
Stoichiometry of Heavy Metal Binding to Peptides Involved in Alzheimer's Disease: Mass Spectrometric Evidence.Adv Exp Med Biol. 2019;1140:401-415. doi: 10.1007/978-3-030-15950-4_23. Adv Exp Med Biol. 2019. PMID: 31347061 Review.
Cited by
-
A Half-Sandwich Os(II) Glucoconjugated NHC Complex as a Modulator of Amyloid Aggregation.Inorg Chem. 2025 Feb 24;64(7):3335-3345. doi: 10.1021/acs.inorgchem.4c04823. Epub 2025 Feb 13. Inorg Chem. 2025. PMID: 39945504 Free PMC article.
-
Peptidomimetics Targeting the Amyloidogenicity of Nucleophosmin 1 Mutations in Acute Myeloid Leukemia.Chembiochem. 2025 Jul 18;26(14):e202500306. doi: 10.1002/cbic.202500306. Epub 2025 Jun 17. Chembiochem. 2025. PMID: 40400267 Free PMC article.
-
Polyoxometalates: metallodrug agents for combating amyloid aggregation.Natl Sci Rev. 2024 Jun 28;11(7):nwae226. doi: 10.1093/nsr/nwae226. eCollection 2024 Jul. Natl Sci Rev. 2024. PMID: 39081537 Free PMC article. Review.
-
A Diruthenium Metallodrug as a Potent Inhibitor of Amyloid-β Aggregation: Synergism of Mechanisms of Action.Inorg Chem. 2024 Jan 8;63(1):564-575. doi: 10.1021/acs.inorgchem.3c03441. Epub 2023 Dec 20. Inorg Chem. 2024. PMID: 38117944 Free PMC article.
-
Nanoscale Structural Characterization of Amyloid β 1-42 Oligomers and Fibrils Grown in the Presence of Fatty Acids.ACS Chem Neurosci. 2024 Sep 18;15(18):3344-3353. doi: 10.1021/acschemneuro.4c00275. Epub 2024 Sep 2. ACS Chem Neurosci. 2024. PMID: 39222387 Free PMC article.
References
-
- Suh J.-M.; Kim G.; Kang J.; Lim M. H.. Strategies employing transition metal complexes to modulate amyloid-β aggregation; ACS Publications: 2018; Vol. 58, pp 8–17. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

