Cytochrome P460 Cofactor Maturation Proceeds via Peroxide-Dependent Post-translational Modification
- PMID: 37338957
- PMCID: PMC10431212
- DOI: 10.1021/jacs.3c03608
Cytochrome P460 Cofactor Maturation Proceeds via Peroxide-Dependent Post-translational Modification
Abstract
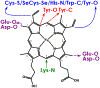
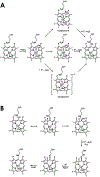
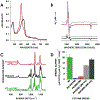
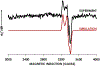
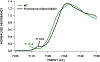
Cytochrome P460s are heme enzymes that oxidize hydroxylamine to nitrous oxide. They bear specialized "heme P460" cofactors that are cross-linked to their host polypeptides by a post-translationally modified lysine residue. Wild-type N. europaea cytochrome P460 may be isolated as a cross-link-deficient proenzyme following anaerobic overexpression in E. coli. When treated with peroxide, this proenzyme undergoes maturation to active enzyme with spectroscopic and catalytic properties that match wild-type cyt P460. This maturation reactivity requires no chaperones─it is intrinsic to the protein. This behavior extends to the broader cytochrome c'β superfamily. Accumulated data reveal key contributions from the secondary coordination sphere that enable selective, complete maturation. Spectroscopic data support the intermediacy of a ferryl species along the maturation pathway.
Figures



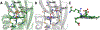

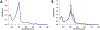
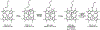
References
-
- Macek B; Forchhammer K; Hardouin J; Weber-Ban E; Grangeasse C; Mijakovic I Protein Post-Translational Modifications in Bacteria. Nat. Rev. Microbiol 2019, 17 (11), 651–664. - PubMed
-
- Walsh CT; Garneau-Tsodikova S; Gatto GJ Jr. Protein Posttranslational Modifications: The Chemistry of Proteome Diversifications. Angew. Chem. Int. Ed 2005, 44 (45), 7342–7372. - PubMed
-
- Lin Y-W The Broad Diversity of Heme-Protein Cross-Links: An Overview. Biochim. Biophys. Acta 2015, 1854 (8), 844–859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

