Diminished responses to mRNA-based SARS-CoV-2 vaccines in individuals with rheumatoid arthritis on immune-modifying therapies
- PMID: 37338983
- PMCID: PMC10445680
- DOI: 10.1172/jci.insight.168663
Diminished responses to mRNA-based SARS-CoV-2 vaccines in individuals with rheumatoid arthritis on immune-modifying therapies
Abstract
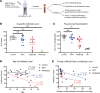
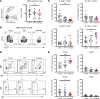
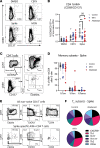
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disorder that causes debilitating swelling and destruction of the joints. People with RA are treated with drugs that actively suppress one or more parts of their immune system, and these may alter the response to vaccination against SARS-CoV-2. In this study, we analyzed blood samples from a cohort of patients with RA after receiving a 2-dose mRNA COVID-19 vaccine regimen. Our data show that individuals on the cytotoxic T lymphocyte antigen 4-Ig therapy abatacept had reduced levels of SARS-CoV-2-neutralizing antibodies after vaccination. At the cellular level, these patients showed reduced activation and class switching of SARS-CoV-2-specific B cells, as well as reduced numbers and impaired helper cytokine production by SARS-CoV-2-specific CD4+ T cells. Individuals on methotrexate showed similar but less severe defects in vaccine response, whereas individuals on the B cell-depleting therapy rituximab had a near-total loss of antibody production after vaccination. These data define a specific cellular phenotype associated with impaired response to SARS-CoV-2 vaccination in patients with RA on different immune-modifying therapies and help inform efforts to improve vaccination strategies in this vulnerable population.
Keywords: Adaptive immunity; Autoimmunity; Costimulation; Vaccines.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

