Biodegradable lipophilic polymeric mRNA nanoparticles for ligand-free targeting of splenic dendritic cells for cancer vaccination
- PMID: 37339211
- PMCID: PMC10293809
- DOI: 10.1073/pnas.2301606120
Biodegradable lipophilic polymeric mRNA nanoparticles for ligand-free targeting of splenic dendritic cells for cancer vaccination
Abstract
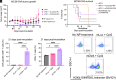
Nanoparticle (NP)-based mRNA cancer vaccines hold great promise to realize personalized cancer treatments. To advance this technology requires delivery formulations for efficient intracellular delivery to antigen-presenting cells. We developed a class of bioreducible lipophilic poly(beta-amino ester) nanocarriers with quadpolymer architecture. The platform is agnostic to the mRNA sequence, with one-step self-assembly allowing for delivery of multiple antigen-encoding mRNAs as well as codelivery of nucleic acid-based adjuvants. We examined structure-function relationships for NP-mediated mRNA delivery to dendritic cells (DCs) and identified that a lipid subunit of the polymer structure was critical. Following intravenous administration, the engineered NP design facilitated targeted delivery to the spleen and preferential transfection of DCs without the need for surface functionalization with targeting ligands. Treatment with engineered NPs codelivering antigen-encoding mRNA and toll-like receptor agonist adjuvants led to robust antigen-specific CD8+ T cell responses, resulting in efficient antitumor therapy in in vivo models of murine melanoma and colon adenocarcinoma.
Keywords: cancer; delivery; mRNA; nanoparticle; vaccine.
Conflict of interest statement
D.M.P. is a consultant for Compugen, Shattuck Labs, Tempest, Immunai, Bristol-Myers Squibb, Amgen, Janssen, Astellas, Rockspring Capital, Immunomic, and Dracen. J.J.G. is a cofounder, manager, and CTO of Dome Therapeutics; cofounder, board member, and CSO of Cove Therapeutics; cofounder and manager of OncoSwitch Therapeutics; cofounder of WyveRNA Therapeutics; scientific advisory board member of Mana Bio; and board member of VasoRx. S.Y.T. is a cofounder and manager of OncoSwitch Therapeutics. D.M.P. owns founder’s equity in manaT Holdings, LLC, Trex, Jounce, Anara, Tizona, Tieza, and RAPT. J.J.G. owns equity in Dome, Cove, OncoSwitch, WyveRNA, Mana Bio, and VasoRx. S.Y.T. owns equity in OncoSwitch. E.B.-A., J.K., S.Y.T., and J.J.G. are a coinventors on patents filed by Johns Hopkins University related to technologies discussed in the manuscript. D.M.P. receives research funding from Compugen, Bristol-Myers Squibb, and Anara and royalties on patents licensed by Compugen, BMS, and Immunomic.
Figures






Similar articles
-
Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses.J Allergy Clin Immunol. 2017 Nov;140(5):1339-1350. doi: 10.1016/j.jaci.2016.12.985. Epub 2017 Mar 23. J Allergy Clin Immunol. 2017. PMID: 28343701 Free PMC article.
-
CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses.Biomaterials. 2015 Feb;40:88-97. doi: 10.1016/j.biomaterials.2014.10.053. Epub 2014 Nov 26. Biomaterials. 2015. PMID: 25465442
-
Toll-like receptor 3-induced immune response by poly(d,l-lactide-co-glycolide) nanoparticles for dendritic cell-based cancer immunotherapy.Int J Nanomedicine. 2016 Nov 2;11:5729-5742. doi: 10.2147/IJN.S109001. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27843314 Free PMC article.
-
Towards Targeted Delivery Systems: Ligand Conjugation Strategies for mRNA Nanoparticle Tumor Vaccines.J Immunol Res. 2015;2015:680620. doi: 10.1155/2015/680620. Epub 2015 Dec 24. J Immunol Res. 2015. PMID: 26819957 Free PMC article. Review.
-
Immune system targeting by biodegradable nanoparticles for cancer vaccines.J Control Release. 2013 Jun 10;168(2):179-99. doi: 10.1016/j.jconrel.2013.03.010. Epub 2013 Mar 21. J Control Release. 2013. PMID: 23524187 Review.
Cited by
-
Potential of Pullulan-Based Polymeric Nanoparticles for Improving Drug Physicochemical Properties and Effectiveness.Polymers (Basel). 2024 Jul 29;16(15):2151. doi: 10.3390/polym16152151. Polymers (Basel). 2024. PMID: 39125177 Free PMC article. Review.
-
Biomimetic noncationic lipid nanoparticles for mRNA delivery.Proc Natl Acad Sci U S A. 2023 Dec 19;120(51):e2311276120. doi: 10.1073/pnas.2311276120. Epub 2023 Dec 11. Proc Natl Acad Sci U S A. 2023. PMID: 38079547 Free PMC article.
-
Targeting Antigen-Presenting Cells to Enhance the Tumor-Spleen Immunity Cycle through Liposome-Neoantigen Vaccine.Adv Sci (Weinh). 2025 May;12(19):e2500021. doi: 10.1002/advs.202500021. Epub 2025 Mar 24. Adv Sci (Weinh). 2025. PMID: 40125791 Free PMC article.
-
Developing mRNA Nanomedicines with Advanced Targeting Functions.Nanomicro Lett. 2025 Feb 21;17(1):155. doi: 10.1007/s40820-025-01665-9. Nanomicro Lett. 2025. PMID: 39979495 Free PMC article. Review.
-
Strategies for non-viral vectors targeting organs beyond the liver.Nat Nanotechnol. 2024 Apr;19(4):428-447. doi: 10.1038/s41565-023-01563-4. Epub 2023 Dec 27. Nat Nanotechnol. 2024. PMID: 38151642 Review.
References
-
- Vormehr M., Türeci Ö., Sahin U., Harnessing tumor mutations for truly individualized cancer vaccines. Annu. Rev. Med. 70, 395–407 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

