Prevalence of Deleterious Variants in MC3R in Patients With Constitutional Delay of Growth and Puberty
- PMID: 37339320
- PMCID: PMC10655545
- DOI: 10.1210/clinem/dgad373
Prevalence of Deleterious Variants in MC3R in Patients With Constitutional Delay of Growth and Puberty
Abstract
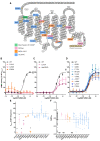
Context: The melanocortin 3 receptor (MC3R) has recently emerged as a critical regulator of pubertal timing, linear growth, and the acquisition of lean mass in humans and mice. In population-based studies, heterozygous carriers of deleterious variants in MC3R report a later onset of puberty than noncarriers. However, the frequency of such variants in patients who present with clinical disorders of pubertal development is currently unknown.
Objective: This work aimed to determine whether deleterious MC3R variants are more frequently found in patients clinically presenting with constitutional delay of growth and puberty (CDGP) or normosmic idiopathic hypogonadotropic hypogonadism (nIHH).
Methods: We examined the sequence of MC3R in 362 adolescents with a clinical diagnosis of CDGP and 657 patients with nIHH, experimentally characterized the signaling properties of all nonsynonymous variants found and compared their frequency to that in 5774 controls from a population-based cohort. Additionally, we established the relative frequency of predicted deleterious variants in individuals with self-reported delayed vs normally timed menarche/voice-breaking in the UK Biobank cohort.
Results: MC3R loss-of-function variants were infrequent but overrepresented in patients with CDGP (8/362 [2.2%]; OR = 4.17; P = .001). There was no strong evidence of overrepresentation in patients with nIHH (4/657 [0.6%]; OR = 1.15; P = .779). In 246 328 women from the UK Biobank, predicted deleterious variants were more frequently found in those self-reporting delayed (aged ≥16 years) vs normal age at menarche (OR = 1.66; P = 3.90E-07).
Conclusion: We have found evidence that functionally damaging variants in MC3R are overrepresented in individuals with CDGP but are not a common cause of this phenotype.
Keywords: MC3R; ALSPAC; UK Biobank; constitutional delay; delayed puberty.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
Comment in
-
Unveiling the Central Regulation of Pubertal Development.J Clin Endocrinol Metab. 2024 Feb 20;109(3):e1307-e1308. doi: 10.1210/clinem/dgad486. J Clin Endocrinol Metab. 2024. PMID: 37589951 No abstract available.
References
-
- Palmert MR, Dunkel L. Delayed puberty. N Engl J Med. 2012;366(5):443‐453. - PubMed
-
- Harrington J, Palmert MR. Distinguishing self-limited delayed puberty from permanent hypogonadotropic hypogonadism: how and why? J Clin Endocrinol Metab. 2021;106(12):e5264‐e5266. - PubMed
-
- Sedlmeyer IL, Palmert MR. Delayed puberty: analysis of a large case series from an academic center. J Clin Endocrinol Metab. 2002;87(4):1613‐1620. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01 HD090071/HD/NICHD NIH HHS/United States
- R01 HD048960/HD/NICHD NIH HHS/United States
- R37 HD043341/HD/NICHD NIH HHS/United States
- MC_PC_15018/MRC_/Medical Research Council/United Kingdom
- MC_UU_00014/1/MRC_/Medical Research Council/United Kingdom
- M01 RR002172/RR/NCRR NIH HHS/United States
- MC_PC_19009/MRC_/Medical Research Council/United Kingdom
- P50 HD104224/HD/NICHD NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- K23 RR015544/RR/NCRR NIH HHS/United States
- MC_UU_00014/5/MRC_/Medical Research Council/United Kingdom
- G9815508/MRC_/Medical Research Council/United Kingdom
- M01 RR000080/RR/NCRR NIH HHS/United States

