Phase 1/2 study of sorafenib added to cladribine, high-dose cytarabine, G-CSF, and mitoxantrone in untreated AML
- PMID: 37339483
- PMCID: PMC10463192
- DOI: 10.1182/bloodadvances.2023010392
Phase 1/2 study of sorafenib added to cladribine, high-dose cytarabine, G-CSF, and mitoxantrone in untreated AML
Abstract
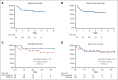
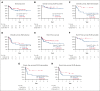
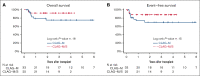
The multikinase inhibitor sorafenib improves event-free survival (EFS) when used with 7 + 3 in adults with newly-diagnosed acute myeloid leukemia (AML), irrespective of the FLT3-mutation status. Here, we evaluated adding sorafenib to cladribine, high-dose cytarabine, granulocyte colony-stimulating factor, and mitoxantrone (CLAG-M) in a phase 1/2 trial of 81 adults aged ≤60 years with newly diagnosed AML. Forty-six patients were treated in phase 1 with escalating doses of sorafenib and mitoxantrone. No maximum tolerated dose was reached, and a regimen including mitoxantrone 18 mg/m2 per day and sorafenib 400 mg twice daily was declared the recommended phase 2 dose (RP2D). Among 41 patients treated at RP2D, a measurable residual disease-negative complete remission (MRD- CR) rate of 83% was obtained. Four-week mortality was 2%. One-year overall survival (OS) and EFS were 80% and 76%, without differences in MRD- CR rates, OS, or EFS between patients with or without FLT3-mutated disease. Comparing outcomes using CLAG-M/sorafenib with those of a matched cohort of 76 patients treated with CLAG-M alone, multivariable-adjusted survival estimates were improved for 41 patients receiving CLAG-M/sorafenib at RP2D (OS: hazard ratio,0.24 [95% confidence interval, 0.07-0.82]; P = .023; EFS: hazard ratio, 0.16 [95% confidence interval, 0.05-0.53]; P = .003). Benefit was limited to patients with intermediate-risk disease (univariate analysis: P = .01 for OS; P = .02 for EFS). These data suggest that CLAG-M/sorafenib is safe and improves OS and EFS relative to CLAG-M alone, with benefits primarily in patients with intermediate-risk disease. The trial was registered at www.clinicaltrials.gov as #NCT02728050.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.B.H. receives research funding from Imago BioSciences, Bayer Pharmaceuticals, Gilead Sciences, Jazz Pharmaceuticals, Incyte Pharmaceuticals, Karyopharm Therapeutics, and Disc Medicine, and has consulted for AbbVie Pharmaceuticals and Notable Labs. E.R.-A. reports travel and conference funding from Jazz Pharmaceuticals, AbbVie, and Gilead. K.-L.A.G. is an employee at WIRB-Copernicus Group. M.-E.M.P. reports research funding from AbbVie, Ascentage, Astex, Biosight, Bristol Myers Squibb (BMS)/Celgene, Cardiff Ongolocy, Glycomimetics, Pfizer, Telios, and Trillium. R.D.C. reports research funding from Amgen, Kite/Gilead, Merck, Pfizer, Servier, and Vanda Pharmaceuticals; receives honoraria/consulting from Amgen, Jazz, Kite/Gilead, and Pfizer; is a member of the data and safety monitoring board for Pepromene Bio; serves on the independent response review committee for Autolus; and his spouse has been employed by and owns stock in Seagen. V.G.O. reports research funding from Pfizer Inc., and consults for Pfizer and Novartis. P.S.B. reports research support GlycoMimetics, Pfizer, Notable Labs, and GPCR, and is a medical adviser for Accordant Health Services/CVS Caremark. J.J.O. receives research funding from Actinium Pharmaceuticals Inc. S.B.K. provides consultancy for Disc Medicine. R.B.W. received laboratory research grants and/or clinical trial support from Amgen, Aptevo, Celgene, ImmunoGen, Janssen, Jazz, Kura, MacroGenics, and Pfizer; has ownership interests in Amphivena; and is (or has been) a consultant to AbbVie, Adicet, Amphivena, BerGenBio, BMS, GlaxoSmithKline, ImmunoGen, Kura, and Orum. None of these disclosures are related to the drugs described in the manuscript (except for the Bayer funding of this research study). The remaining authors declare no competing financial interests.
The current affiliation for P.S.B. is Division of Leukemia, Department of Hematology & Hematopoietic Cell Transplantation, City of Hope, Duarte, CA.
Elihu H. Estey died on 8 October 2021.
Figures

References
-
- Shimony S, Stahl M, Stone RM. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(3):502–526. - PubMed
-
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345–1377. - PubMed
-
- Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5(10):835–844. - PubMed
-
- Röllig C, Serve H, Hüttmann A, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2015;16(16):1691–1699. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

