Detection of Substandard and Falsified Antibiotics Sold in the Democratic Republic of the Congo Using Validated HPLC and UV-Visible Spectrophotometric Methods
- PMID: 37339757
- PMCID: PMC10397439
- DOI: 10.4269/ajtmh.23-0045
Detection of Substandard and Falsified Antibiotics Sold in the Democratic Republic of the Congo Using Validated HPLC and UV-Visible Spectrophotometric Methods
Abstract
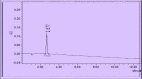
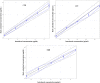
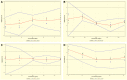
The access to afford safe, effective, and genuine medications is a major challenge for people in low- to middle-income countries. This study aimed at developing and validating simple, accurate, and inexpensive analytical liquid chromatography and ultraviolet-visible spectrophotometric methods to ensure quality control of antibiotics sold in formal and informal pharmaceutical markets. It focused on four antibiotics (azithromycin [AZT], cefadroxil [CFD], cefixime [CFX], and erythromycin [ERH]) used to treat infectious diseases in the region of Haut-Katanga in the Democratic Republic of the Congo (DRC). The total error strategy (accuracy profile) matching with the validation requirements of International Council on Harmonization was used for the validation. The validation results showed that three analytical methods of AZT, CFD, and ERH were validated according to the accuracy profile obtained, whereas the proposed method of CFX was not validated. Therefore, the United State Pharmacopoeia method permitted to quantify CFX samples. The dosage intervals ranged from 25 to 75 µg/mL for CFD, from 750 to 1,500 µg/mL for AZT, and from 500 to 750 µg/mL for ERH. The application of the validated method to samples collected (N = 95) allowed the detection of 25% substandard antibiotics with a rate of poor quality much higher in the informal circuit compared with the formal one (54% versus 11%; P < 0.05). The routine application of these methods will strengthen the quality control of drugs marketed in DRC. This study gives evidence for the availability of poor-quality antibiotics in the country, requiring the immediate attention of the national medicine regulatory authority.
Figures

References
-
- WHO , 2017. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products. Geneva, Switzerland: World Health Organization.
-
- Peyraud N. et al., 2017. An epidemic of dystonic reactions in central Africa. Lancet Glob Health 5: e137–e138. - PubMed
-
- Schneider M, Nam NHT, 2021. How pharmaceutical companies can prevent falsified medicine and vaccines from entering African markets. J Intellect Prop Law Pract 16: 907–915.
-
- Borse NN. et al., 2021. Responding to the Surge of Substandard and Falsified Health Products Triggered by the Covid-19 Pandemic. Available at: https://www.usp.org/sites/default/files/usp/document/our-impact/covid-19.... Accessed January 30, 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

